PROSPECTIVE ARNI VERSUS ACE INHIBITOR TRIAL TO DETERMINE SUPERIORITY IN REDUCING HEART FAILURE EVENTS AFTER MYOCARDIAL INFARCTION (PARADISE-MI)
Pfeffer M, presented a study in a session at the American College of Cardiology (ACC) 2021 scientific sessions: virtual congress. A prospective angiotensin receptor-neprilysin inhibition (ARNI) versus angiotensin-converting enzyme (ACE) inhibitor study is being conducted to evaluate which is superior in terms of lowering heart failure. Prospective ARNI versus ACE inhibitor trial aimed to determine superiority in reducing heart failure Events after Myocardial Infarction (PARADISE-MI) is a randomised, active-controlled, global (41 country) study of acute MI (AMI) patients (within 0.5-7 days).
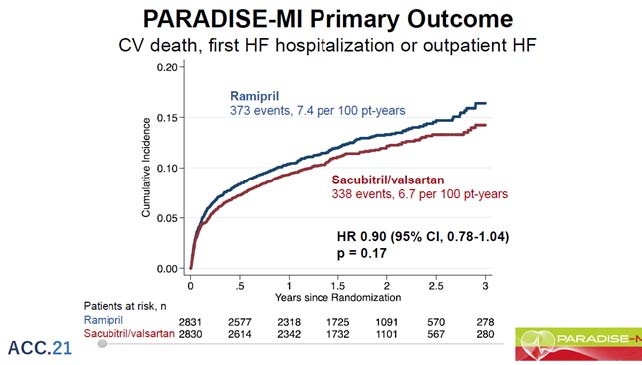
The study’s main goal was to see if sacubitril/valsartan was more successful than a well-known00 ACE inhibitor in lowering the rate of cardiovascular (CV) mortality, heart failure (HF) hospitalisation, and the development of symptomatic HF. The trial was event-driven, with about 708 patients experiencing the key event, providing 80% power to detect a 19% reduction in relative risk.
The randomization of 5669 patients took place 4.3 days and 1.8 days after their first AMI presentation. The average age was 64 years and 12 months, with women accounted for 24% of the participants. In 76% of cases, there was ST-segment elevation; in 87% of cases, total percutaneous coronary intervention was done, and thrombolysis was administered in 5% of cases. The left ventricular ejection fraction (LVEF) was 37.9%, and 58% of the patients were classified as Killip class 2. The protocol-designed number of main events happened during a median follow-up of 20 months. For the first time, the primary endpoint as well as adverse events for sacubitril/valsartan versus ramipril was being reported.
Predetermined decreases in both investigator reports of the primary composite as well as total (recurrent) adjudicated events indicate sacubitril/incremental valsartan’s therapeutic advantages. Sacubitril/safety valsartan’s and tolerability in this AMI group was equivalent to that of the ACEi.
ASPIRIN DOSING: A PATIENT-CENTRIC TRIAL ASSESSING BENEFITS AND LONG-TERM EFFECTIVENESS TRIAL (ADAPTABLE)
Jones WS, presented a study in a session at the American College of Cardiology (ACC) 2021 scientific sessions: virtual congress. ADAPTABLE is a randomised, open-label pragmatic trial that compares the efficacy and safety of 81 mg of daily aspirin to 325 mg of daily aspirin in people with atherosclerotic cardiovascular disease.
A total of 15,076 people were enrolled in the study, which took place at 40 different locations across the United States. A known diagnosis of atherosclerotic cardiovascular disease, past myocardial infarction, and/or previous percutaneous coronary intervention or surgical revascularization was all important inclusion criteria.
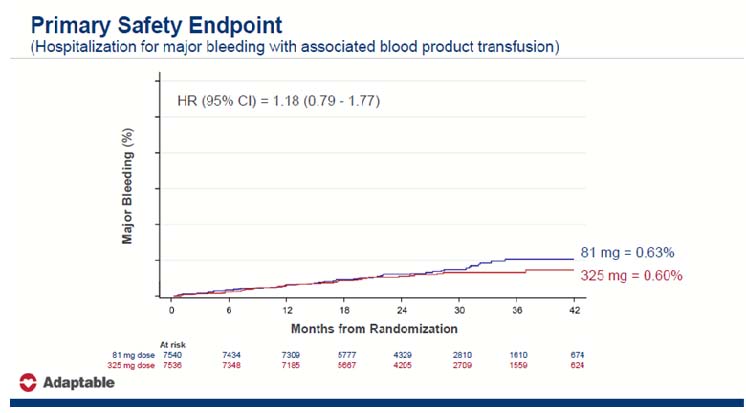
Age > 65 years, creatinine > 1.5 mg/dL, diabetic mellitus, current cigarette smoking, cerebrovascular illness, peripheral artery disease, heart failure, left ventricular ejection fraction 50%, systolic blood pressure > 140 mmHg, or LDL cholesterol > 130 mg/dL were all essential enrichment criteria. The primary efficacy endpoint was a composite of all-cause mortality, myocardial infarction hospitalisation, and stroke hospitalisation. Hospitalization for serious bleeding with a blood product transfusion was the primary safety objective.
The study lasted from April 2016 to June 2019, with a median follow-up of 21.7 months. The last study queries from the PCORnet Common Data Model, Centers for Medicare and Medicaid Services claims data, and commercial health insurance claims data began in November 2020, after research participation concluded in June 2020. There was no difference in mortality, MI, or stroke in those who received 81 mg vs. 325 mg. There was a difference in adherence to the study dose/intervention (the 325 mg group had greater dosage switching).
It was concluded that patients did not stay on the 325 mg dosage for a variety of reasons includes tolerability, medical reasons, participant preferences and clinician practices.
ORAL ANTI-XA ANTICOAGULATION AFTER TRANS-AORTIC VALVE IMPLANTATION FOR AORTIC STENOSIS: THE RANDOMIZED ATLANTIS TRIAL
Collet J, presented a study in a session at the American College of Cardiology (ACC) 2021 scientific sessions: virtual congress. The significance of chronic anticoagulation medication vs antiplatelet treatment is still debatable, especially in the case of Non-vitamin K Oral Anti-Coagulants (NOAC), which have demonstrated to be ineffective in patients with prosthetic heart valves. In individuals with atrial fibrillation, however, NOACs have demonstrated to be more effective than vitamin K antagonists (VKA).
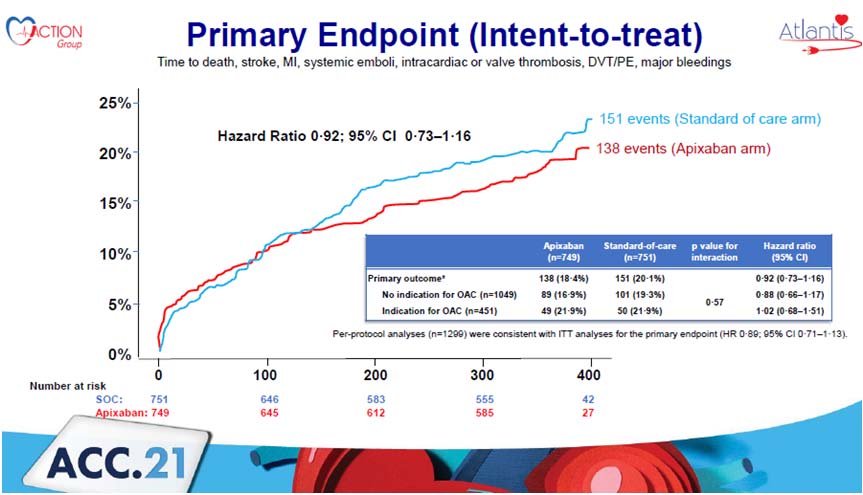
The global randomised open-label ATLANTIS research compared standard of care (SOC Group) to an apixaban-based treatment after successful transcatheter aortic valve replacement (TAVR) (Anti-Xa Group). The randomization was stratified based on the need for long-term anticoagulant therapy for reasons other than the TAVR procedure. Between August 2016 and June 2019, a total of 1510 patients were randomised, with a one-year follow-up. 138 incidents of all-cause mortality, TIA/stroke, myocardial infarction, valve thrombosis, pulmonary embolism, deep venous thrombosis, systemic embolism, or significant bleeding were reported in the Apixaban group against 151 occurrences in the standard of care arm.
In the secondary outcome, the Apixaban group had 0.1% less Deep vein thrombosis or pulmonary embolism compared to 1.5% in the standard of care group. Apixaban reduces subclinical valve thrombosis (albeit not statistically), a reduction caused by the stratum of individuals without a reason for anticoagulation. Only in the strata of individuals without a reason for anticoagulation is there a signal on non-cardiovascular mortality when compared to antiplatelet medication.
In conclusion, the frequency of non-cardiovascular deaths in the group without anticoagulant indications was minimal, according to the researchers, who noted that the mortality were mostly caused by sepsis or acute renal failure and were very occasionally preceded by mild bleeding episodes.
SEX-SPECIFIC OUTCOMES IN HIGH-RISK PATIENTS RECEIVING TICAGRELOR WITH OR WITHOUT ASPIRIN AFTER PERCUTANEOUS CORONARY INTERVENTION: RESULTS FROM THE TWILIGHT STUDY
Huber K, presented a study in a session at the American College of Cardiology (ACC) 2021 scientific sessions: virtual congress. A three-month course of dual antiplatelet treatment (DAPT) followed by monotherapy with a strong P2Y12 inhibitor lowers bleeding without increasing ischemic events after percutaneous coronary intervention (PCI). It’s unclear whether these therapy effects are heterogeneous based on gender.
Ticagrelor with Aspirin or Alone in High-Risk Patients after Coronary Intervention (TWILIGHT) was a randomised controlled study that compared Ticagrelor plus placebo to Ticagrelor plus aspirin in a PCI group with a high risk of bleeding or ischemic events after three months of DAPT. At one year, the Bleeding Academic Research Consortium type 2, 3, or 5 was the main outcome. Death, myocardial infarction, and stroke were among the ischemic outcomes. Patients were sorted by sex for this predetermined secondary analysis.
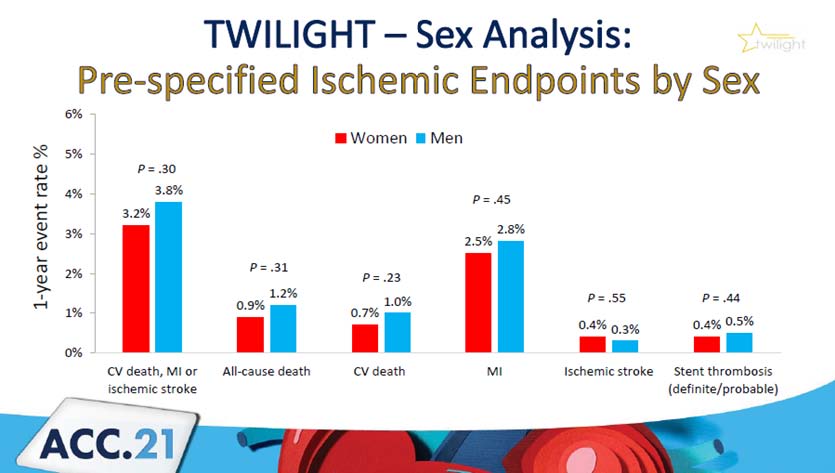
A total of 9006 patients were enrolled, with 7119 randomly assigned. Women made up 23.9% (n=1698) of the randomised patients. After multivariable correction, there was no significant difference between men and women in terms of the major bleeding endpoint (hazard ratio 1.20; 95% confidence range 0.95 – 1.52; p=0.119). Furthermore, no significant sex differences in ischemia outcomes were discovered.
In the TWILIGHT study, women had a larger risk of bleeding than males, but ischemia episodes occurred at equal rates. In women and men, the benefits of early aspirin cessation in reducing bleeding without increasing ischemic events were equal.
TRANSFEMORAL TRICUSPID VALVE REPLACEMENT IN PATIENTS WITH TRICUSPID REGURGITATION: 30-DAY RESULTS OF THE TRISCEND STUDY
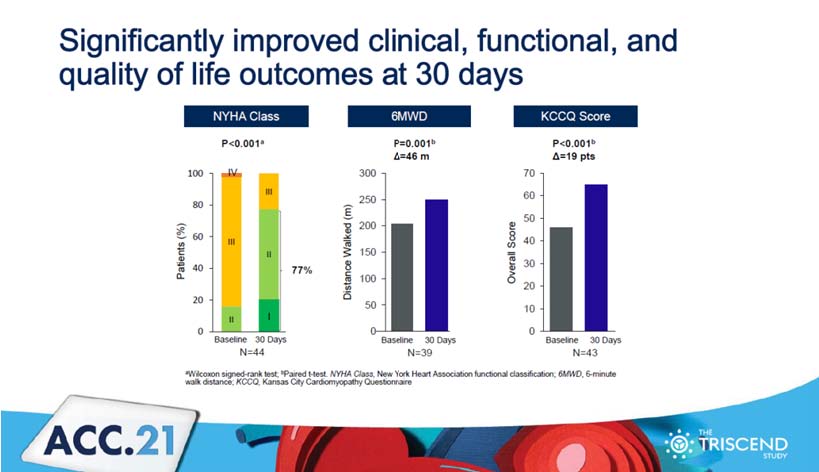
Kodali SK, presented a study in a session at the American College of Cardiology (ACC) 2021 scientific sessions: virtual congress. The prospective, single-arm, multicenter TRISCEND research looked at 56 patients who had their tricuspid valves replaced transcatheter (EVOQUE system, Edwards Lifesciences, Irvine, CA). The participants were monitored for 30 days and had a mild TR. Oversight was provided by a data safety monitoring board, an echocardiographic core lab, and a clinical events committee.
The average age was 79 years old, with 77% of the participants being female. Patients had TR ≥ severe (92%), atrial fibrillation (91%), and New York Heart Association (NYHA) class III/IV (84%). The mortality risk score (MV Repair) of the Society of Thoracic Surgeons was 7.7%. A pacemaker or ICD was worn by 34% of those surveyed. TR was decreased to mild in 98% of cases after 30 days. Almost 1 rate of cardiovascular mortality, 2 non-elective tricuspid re-interventions, 1 major access site/vascular complication, and 12 serious bleeding episodes comprised the 22.6% composite major adverse event rate (none life-threatening or fatal). The NYHA improved (p0.001), with 77% of students in class I/II. The 6-minute walk distance increased by 46 metres (p=0.001), whereas the Kansas City Cardiomyopathy Questionnaire score increased by 19 points (p<0.001).
In patients with clinically severe TR, treatment with EVOQUE through a transfemoral technique revealed technical feasibility, safety, and symptomatic improvement after 30 days.
REDUCTIONS IN TOTAL ISCHEMIC EVENTS WITH RIVAROXABAN IN PATIENTS WITH SYMPTOMATIC PAD AFTER REVASCULARIZATION: THE VOYAGER PAD TRIAL
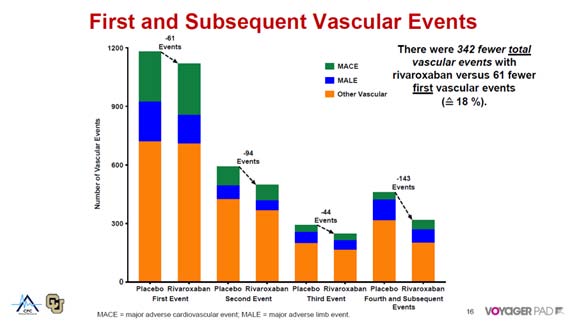
Bauersachs R, presented a study in a session at the American College of Cardiology (ACC) 2021 scientific sessions: virtual congress. Lower extremity revascularization (LER) patients with peripheral arterial disease (PAD) are at a significant risk of significant adverse limb and cardiovascular (CV) events. In the vascular outcomes study of acetylsalicylic acid (ASA) along with rivaroxaban in endovascular or surgical limb revascularization for peripheral artery disease (VOYAGER PAD) trial, rivaroxaban 2.5 mg twice daily plus aspirin was found to lower first occurrences by 15% compared to aspirin alone.
The VOYAGER PAD study assigned PAD patients receiving LER to rivaroxaban 2.5 mg twice day or placebo on a background of aspirin 100 mg daily in a double-blind, placebo-controlled study. Time to first event in a composite of acute limb ischemia, major amputation due to a vascular aetiology, myocardial infarction, ischemic stroke, or CV mortality was the main objective. A shared log-normal frailty model was used to estimate hazard ratios in the current total events study.
There were 1,092 initial and 522 subsequent primary events among 6,564 randomised individuals. Rivaroxaban reduced the number of initial incidents by 15% (HR 0.85, 95% CI 0.76 – 0.96, p=0.0085; 76 first events avoided). Rivaroxaban also decreased the total number of incidents (HR 0.82, 95% CI 0.71 – 0.94, p=0.0049; 124 total events avoided). The overall number of events avoided with rivaroxaban 2.5 mg twice day with aspirin in patients with symptomatic PAD undergoing LER was nearly double the number of initial events avoided.
When compared to aspirin alone, rivaroxaban 2.5 mg twice daily with aspirin lowers first and subsequent adverse limb and cardiovascular events, with an even higher overall benefit when all events are included. It should also be considered as an additional treatment following LER to lessen the severity of the first and recurrent unfavourable consequences.
SAFETY OF CLOPIDOGREL VERSUS TICAGRELOR IN STABILIZED PATIENTS WITH ACUTE MYOCARDIAL INFARCTION AFTER PERCUTANEOUS CORONARY INTERVENTION: TALOS-AMI TRIAL
Chang K, presented a study in a session at the American College of Cardiology (ACC) 2021 scientific sessions: virtual congress. The ischemic risk is higher in the early stages of percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI), although the bleeding risk is still significant throughout the maintenance phase.
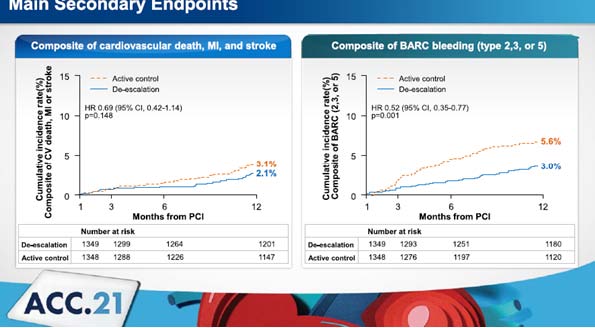
Patients were randomly allocated to either Aspirin 100 mg plus Clopidogrel 75 mg daily Clopidogrel or aspirin 100 mg plus Ticagrelor 90 mg twice daily in a 1:1 ratio in this multicentre, randomised, open-label trial enrolling AMI patients with no adverse events during the first month following an index PCI. From 1 to 12 months following an index PCI, the main outcome was a composite of cardiovascular mortality, MI, stroke, or bleeding type 2, 3, or 5 according to Bleeding Academic Research Consortium (BARC) criteria. In the intention to treat population (ITT) population, a non-inferiority test was conducted first, followed by a superiority test if the difference was substantial.
A total of 2697 patients were allocated to one of two groups: Clopidogrel (1349 patients) or Ticagrelor (1349 patients) (1348 patients). The main objectives were met in 59 patients (4.8%) in the Clopidogrel group and 104 patients (8.5%) in the Ticagrelor group after one year (p 0.001 for non-inferiority,
p< 0.001 for superiority). The Ticagrelor group had a considerably higher risk of bleeding (p = 0.001), but there was no change in the ischemia risk (p = 0.149).
The de-escalating DAPT method switching from Ticagrelor to Clopidogrel at 1 month significantly lowered the likelihood of net clinical outcomes in stable AMI patients, owing to a decrease in bleeding.
ENDOVASCULAR ULTRASOUND RENAL DENERVATION TO TREAT HYPERTENSION RESISTANT TO A FIXED DOSE TRIPLE MEDICATION PILL: THE RANDOMIZED SHAM-CONTROLLED RADIANCE-HTN TRIO TRIAL
Kirtane A, presented a study in a session at the American College of Cardiology (ACC) 2021 scientific sessions: virtual congress. Endovascular renal denervation (RDN) lowers blood pressure (BP) in individuals with mild-moderate hypertension, but its BP-lowering efficacy in individuals with resistant hypertension has yet to be shown.
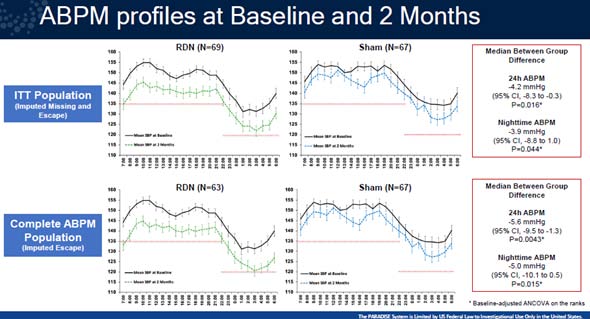
In a multicenter, blinded, randomised, sham-controlled trial, patients with hypertension resistant to at least three antihypertensive medicines, including a diuretic, were included. Subjects with daytime ambulatory BP of 135/85 mmHg were randomised to ultrasound RDN or a sham treatment after 4 weeks of this standardised treatment. The change in daytime ambulatory systolic blood pressure (dASBP) in the intention-to-treat (ITT) group at 2 months was the main outcome.
A total of 136 people were randomly assigned to either the RDN (N=69) or the sham treatment (N=67). At two months, urine samples revealed that adherence to the combo tablet was above 80%. In the ITT population, RDN (-8.0 mmHg vs. -3.0 mmHg; median between-group difference -4.5 mmHg; 95% CI -8.5 to -0.3 mmHg, adjusted p=0.022) lowered dASBP more than the sham treatment (-8.0 mmHg vs. -3.0 mmHg; median between-group difference -4.5 mmHg; 95% CI The reduction in dASBP was -9.7 mmHg in the RDN group (n=63) vs. -3.0 mmHg in the sham group (n=67) among participants with ambulatory BP data at baseline and follow-up; median difference -5.8 mmHg; 95% CI -9.7 to -1.6 mmHg, adjusted p=0.005. In the ITT group, RDN lowered 24-hour SBP more than sham (median difference -4.2 mmHg; 95% CI -8.3 to -0.3 mmHg, adjusted p=0.016), with similar reductions in night time (p=0.044) and office (p=0.037). The RDN group had one access site pseudo aneurysm, no renal artery stenosis, and no change in renal function across groups after two months.
In participants with hypertension resistant to a standardised single pill combination of 3 guideline-recommended medications, ultrasound RDN safely decreased blood pressure when compared to a sham procedure.
FRACTIONAL FLOW RESERVE-GUIDED VERSUS ANGIO-GUIDED MULTIVESSEL REVASCULARIZATION IN ST-ELEVATION MYOCARDIAL INFARCTION PATIENTS: THE FLOWER-MI RANDOMIZED TRIAL
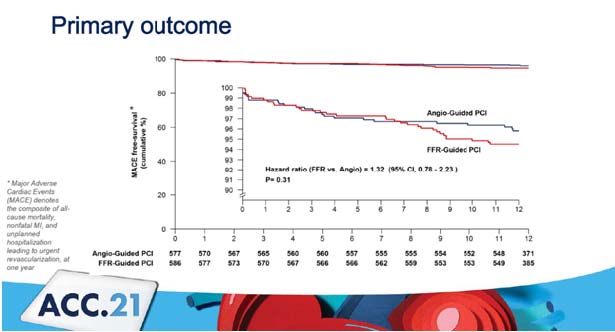
Puymirat E, presented a study in a session at the American College of Cardiology (ACC) 2021 scientific sessions: virtual congress. A randomised, multicenter (40 locations in France) experiment was done in 1171 patients who had successful initial percutaneous coronary intervention (PCI) and 50% stenosis in 1 additional non-culprit lesion appropriate for PCI.
Patients were randomly assigned to have either full revascularization of non-infarct-related arteries guided by FFR (590 patients) or angiography (590 patients) (581 patients). Non-culprit lesions PCI was performed at the index operation or during a staged procedure before discharge (5 days). At 12 months, the primary outcome was a composite of all-cause mortality, nonfatal myocardial infarction (MI), and unexpected hospitalisation with urgent revascularization. Secondary outcomes were cost-effectiveness and cost utility after a year.
The average age was 62.11 years; with 83% of men having diabetes and 16% having an anterior MI. PCI was performed on non-culprit lesions in 96.2% of cases during a phased procedure. In the angio-guided group, the number of operations was 2.48±0.85, while in the fractional flow reserve (FFR)-guided group, it was 2.07±0.99. The average length of follow-up was 12 months, with 6 patients (0.5%) missing out. Almost 4.2 events of Major Adverse Cardiac Events (MACE) were reported in the angio-guided group vs. 5.5 in the FFR-guided group. 1.7 events of MI was reported in the angio-guided group vs. 3.1 in the FFR-guided group. Angio-guided PCI provides higher benefit in cost effectiveness and cost utility than the FFR-guided group.
When compared to the angio-guided group, FFR-guided PCI of non-infarct-related lesions has no effect on the risk of mortality, reinfarction, or urgent revascularization after one year.
ASPIRIN VS. CLOPIDOGREL DURING CHRONIC MAINTENANCE MONOTHERAPY AFTER PERCUTANEOUS CORONARY INTERVENTION: THE HOST EXAM RANDOMIZED CONTROLLED TRIAL
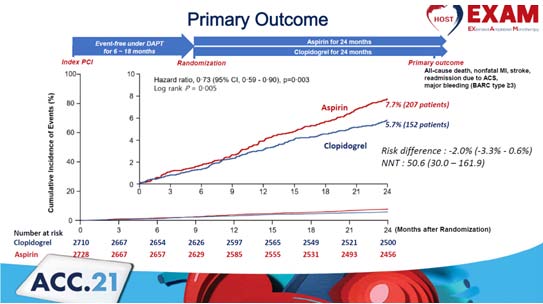
Kim H, presented a study in a session at the American College of Cardiology (ACC) 2021 scientific sessions: virtual congress. After percutaneous coronary intervention (PCI), guidelines recommend 6- to 12-months of dual antiplatelet treatment (DAPT). However, beyond the DAPT length, the best single antiplatelet drug is unknown.
This research is a multicenter, prospective, randomised, open-label, comparative efficacy experiment. Patients with no clinical events for 12 to 6 months after index PCI were randomly allocated to either the aspirin or Clopidogrel monotherapy group in a 1:1 ratio. The working hypothesis is that in 1-year event-free patients following PCI, long-term antiplatelet monotherapy with Clopidogrel will have better outcomes than aspirin.
At 2 years following randomization, the primary objective is composite clinical events, which are defined as all-cause mortality, non-fatal myocardial infarction, stroke, readmission owing to acute coronary syndrome, and serious bleeding events (defined as Bleeding Academic Research Consortium class 3 events).
From March 2014 to May 2018, a total of 5,436 patients from 37 sites were randomly allocated and had the following profiles: mean age 63.5 ±10.7 years, 74.5% of whom were male, 34.2% of whom had diabetes, and 12.7% of whom had chronic renal disease. At 2 years, Group X had a lower rate of the major composite endpoint (6.1% vs. 8.2 Hazard ratio 0.73, 95% confidence interval 0.60 to 0.90, p=0.004) than Group Y 6.1% vs. 8.2%, Hazard ratio 0.73, 95% confidence interval 0.60 to 0.90, p=0.004). In Group X, both thrombotic and severe bleeding events (cardiac mortality, nonfatal myocardial infarction, stroke, readmission due to acute coronary syndrome, and stent thrombosis) were considerably lower than in Group Y.
Clopidogrel monotherapy compared to Aspirin significantly decreased the risk of adverse clinical events in chronic maintenance monotherapy after 1 year of DAPT after PCI.
IMPACT OF COMPLETENESS OF REVASCULARIZATION ON CLINICAL OUTCOMES IN PATIENTS WITH STABLE ISCHEMIC HEART DISEASE TREATED WITH INVASIVE VS. CONSERVATIVE STRATEGY: THE ISCHEMIA TRIAL
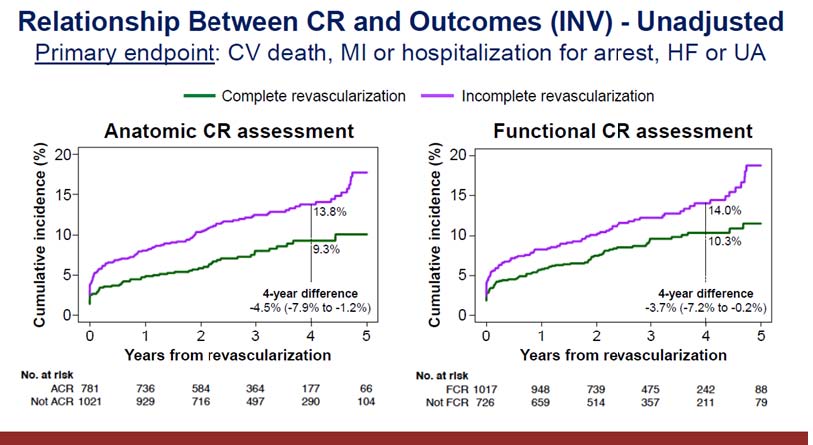
Stone G, presented a study in a session at the American College of Cardiology (ACC) 2021 scientific sessions: virtual congress. International Study of Comparative Health Effectiveness With Medical and Invasive Approaches (ISCHEMIA) Trial randomised 5179 patients with stable coronary artery disease (CAD) and at least mild ischemia to an invasive strategy (INV) of angiography with PCI or CABG if possible + optimum medical treatment (OMT) vs. a conservative strategy (CON) of OMT alone at 320 locations in 37 countries. The composite rate of CV mortality, MI, or hospitalisation for unstable angina, heart failure, or resuscitated cardiac arrest was the main objective. CV death or MI was the primary secondary clinical objective.
In all INV patients, a 3-vessel angiographic core lab study was conducted. The impact of anatomic complete revascularization and functional complete revascularization (ACR and FCR) in patients in the INV vs. CON groups will be examined in uncorrected and adjusted analyses utilising propensity matched and inverse probability weighted methods, with data from blinded CTA to match baseline CAD extent.
A complete revascularization rate of 43.4% was recorded during ACR evaluation vs. 58.3% during FCR evaluation among 1825 individuals randomised to INV who had a revascularization procedure done within 6 months prior to a primary endpoint event. Complete revascularization had a cumulative incidence of 9.3%, while incomplete revascularization had a cumulative incidence of 13.8%. After 5 years of follow-up, the INV approach had 318 primary endpoint events compared to 352 for the CON approach. At the ACR evaluation, 43.6% of the 2296 patients assigned to INV reported full revascularization, compared to 58.5% during the FCR evaluation. In INV-ACR, 11.9% of primary endpoint events were reported as compared to 15.4% in CON. In INV-FCR, 13.1% of main endpoint events were recorded compared to 15.4% in CON.
According to the current findings, establishing anatomic CR may improve them results of an INV method in patients with CCD and moderate ischemia. When deciding between an INV and a CON strategy in patients with CCD, the likelihood of achieving anatomic CR safely should be considered.
SACUBITRIL/VALSARTAN (LCZ696) IN PATIENTS WITH ADVANCED HEART FAILURE AND REDUCED EJECTION FRACTION: RESULTS OF THE LIFE TRIAL
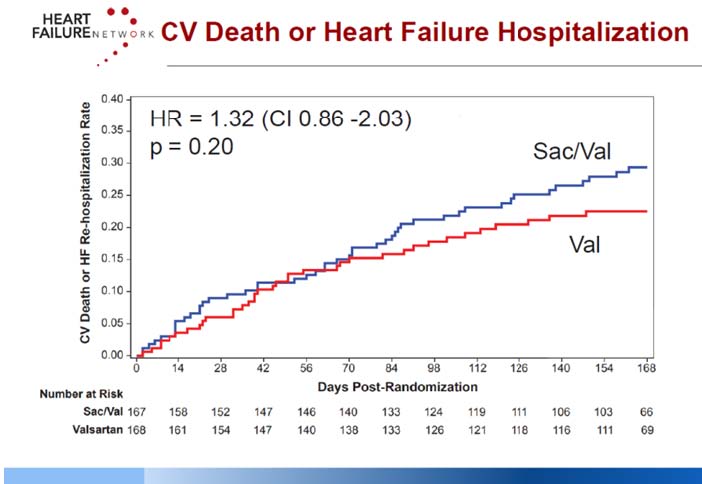
Mann DL, presented a study in a session at the American College of Cardiology (ACC) 2021 scientific sessions: virtual congress. The Losartan Intervention For Endpoint Reduction Trial (LIFE trial) study is a 24-week prospective, multicentre, double-blind, active-comparator trial to assess the efficacy, safety, and tolerability of Sacubitril/valsartan (Sac/Val) compared with valsartan (Val) in patients with advanced HF with reduced ejection fraction (HFrEF), Planned sample size was 400 randomized (1:1) patients. Key entry criteria included systolic blood pressure > 90 mmHg, EF >35%, estimated glomerular filtration rate > 20 mL/min/1.73 m2, NYHA class IV symptoms and natriuretic peptide concentration (B-type natriuretic peptide [BNP] ≥250 pg/mL or N-terminal-proBNP [NT-proBNP] ≥800 pg/mL). The proportionate change from baseline in the area under the curve for NT-proBNP levels (Core Laboratory) assessed through week 24 is the main goal. Clinical results, safety, and tolerability are examples of secondary and tertiary objectives.
Due to the COVID-19 pandemic, enrollment in the LIFE study was discontinued on March 23, 2020. The primary analyses were limited to 335 participants who were randomised on or before December 7, 2019, and who had a Week 12 visit before March 1, 2020, according to the revised LIFE statistical analysis strategy.
Thirty patients who were randomly assigned after December 7, 2019 will be included in secondary analysis. On 12/11/20, patient follow-up and database finalisation were accomplished. Through 24 weeks, neither the Sac/Val nor the Val treatments lowered median NT proBNP levels below baseline. Sac/Val reported 103.2 events efficacy endpoints as compared to 111.2 with Val. Hypotension, poor renal function, and hyperkalemia were recorded in 17%, 4%, and 17% of Sac/Val patients, respectively, compared to 12%, 4%, and 9% of Val patients, respectively.
In terms of reducing NT proBNP levels, Sac/Val was not superior to Val. The clinical composite of days alive, out of the hospital, and free of HF episodes was not improved by adding Sac/Val.
A RANDOMIZED ABLATION-BASED ATRIAL FIBRILLATION RHYTHM CONTROL VERSUS RATE CONTROL TRIAL IN PATIENTS WITH HEART FAILURE AND HIGH BURDEN ATRIAL FIBRILLATION (RAFT-AF)
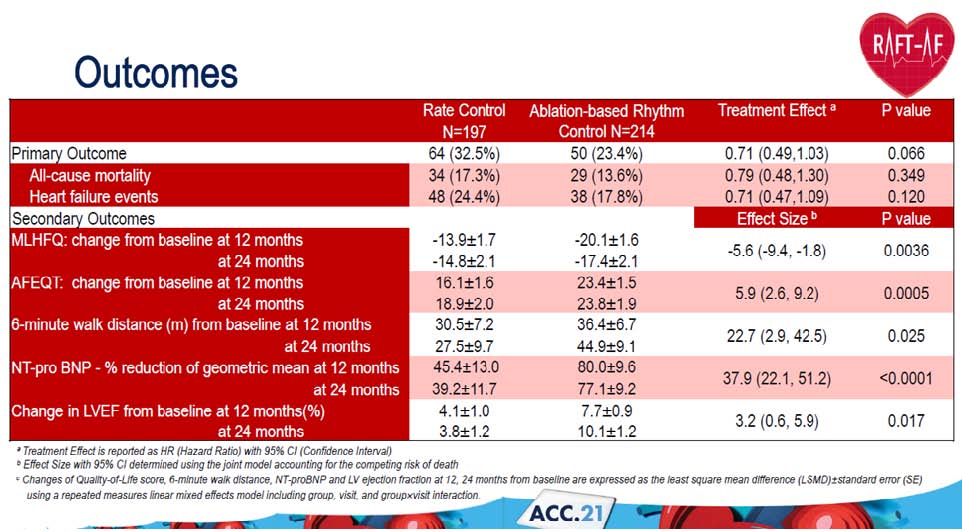
Tang ASL, presented a study in a session at the American College of Cardiology (ACC) 2021 scientific sessions: virtual congress. This is a multicentre, multinational, randomised open-label experiment with blinded outcome evaluation. Patients with NYHA class II-III heart failure (HF), either decreased or retained ejection fraction, or high load atrial fibrillation (AF), were randomly assigned to rate control or ablation-based rhythm control in a 1:1 ratio. Drugs, atrioventricular node (AVN) modification, and bi-ventricular pacing were used to maintain a resting heart rate of 80 beats per minute and a 6-minute walk heart rate of 110 beats per minute. The pulmonary vein isolation and extra ablation lesions used in ablation-based rhythm regulation. The primary outcome was a composite of all-cause death and HF-related hospitalisation. At 12 and 24 months, secondary outcomes include mortality from HF, change in left ventricular ejection fraction (LVEF), N-terminal-proBNP (NT-proBNP), 6 minute walk distance, and quality of life.
A total of 411 patients were enrolled at 21 centres. At baseline, the mean age was 67±8 years; 26% were women; NYHA Class II in 67%, Class III in 33%; 7% had high burden paroxysmal atrial fibrillation (AF), 93% has persistent AF; 58% had LVEF ≤ 45%, 42% had LVEF > 45%, median NT-proBNP was 1619 (1006,2693) pg/ml. Median follow up was 3.2 years. 17.3% of all-cause mortality was reported in rate control vs. 23.4% in ablation-based rhythm control. 17.8% in ablation-based rhythm control vs. 24.4% heart failure events was reported in rate control. 44.9 m change in 6 minute walk distance from baseline at 24 months in ablation-based rhythm control and 27.5 m change in 6 minute walk distance from baseline at 24 months in rate control group.
Patients in the ablation-based rhythm control group had numerically fewer primary outcome events, and greater improvement in LV function, improvement of quality of life and reduction of NT-proBNP than patients in the rate-control group.
HYPERINVASIVE APPROACH IN REFRACTORY OUT-OF-HOSPITAL CARDIAC ARREST: AN OPEN-LABEL RANDOMIZED CONTROLLED TRIAL. PRAGUE OHCA STUDY
Belohlavek J, presented a study in a session at the American College of Cardiology (ACC) 2021 scientific sessions: virtual congress. A randomised controlled study was done in out-of-hospital cardiac arrest (OHCA) to compare the hyperinvasive (H) method (early transfer, extracorporeal membrane oxygenation (ECPR), and immediate invasive examination) to standard (S) care.
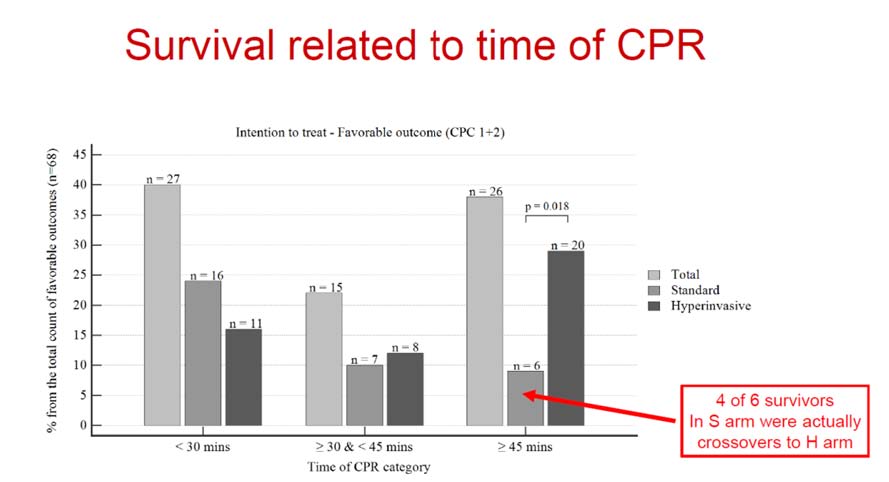
Adults under the age of 18 who were resuscitated for OHCA but did not have spontaneous circulation in either the H or S arm were randomised. Mechanical compression was started in the H arm, followed by rapid transfer to a cardiac facility, where ECPR was started following an examination of inclusion and exclusion criteria. Standard advanced cardiac life support was maintained on site in the S arm. In both arms, post-resuscitation treatment was standardised. The primary goal was to have a 6-month survival rate with a favourable neurological prognosis (Cerebral Performance Category of 1-2). The 30-day neurological and cardiac recovery was secondary outcomes.
256 out of 4345 OHCA patients were randomised between March 2013 and October 2020, 132 in the S group and 124 in the H group. Eleven (8.3%) patients in the S group were transferred to the H group and got ECPR, whereas nine (7.2%) patients in the H group were transferred to the S group. In the S and H groups, resuscitation lasted 46.0 [42.3-53.5] and 58.0 [52.2-60.0] minutes, respectively (p=0.04). In an intention-to-treat analysis, 39 (31.5%) of the H patients and 29 (22%) of the control patients met the main outcome (OR: 1.63 [95% CI 0.93- 2.85]; p=0.09). While 5 out of 11 patients who were assigned to the S group and then switched to the H strategy had a good neurological result, no patient who was allocated to the H group and then switched to the S arm survived (p=0.02). At 30 days, the H group had a higher rate of neuro-recovery (43/124 (34,7% ) vs. 30/132 (22,7% ), OR: 1.81 [95% CI 1.04- 3.13]; p=0.03, but not cardiac (54/124 (43,5% ) vs. 45/132 (34,1% )); OR: 1.49 [95% CI 0.91- 2.47]; p=0.12).
When compared to normal advanced cardiovascular life support (ACLS), resuscitation for OHCA utilizing a hyperinvasive technique involving ECPR did not substantially enhance neurologically positive outcomes at 6 months.
THERAPEUTIC HYPOTHERMIA FOLLOWING OUT-OF-HOSPITAL CARDIAC ARREST: A RANDOMIZED TRIAL COMPARING MILD AND MODERATE THERAPEUTIC HYPOTHERMIA
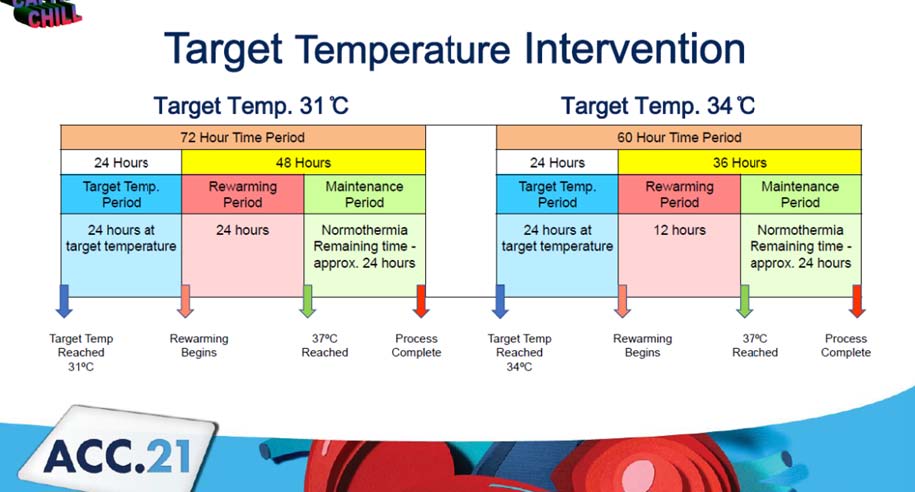
May MRL, presented a study in a session at the American College of Cardiology (ACC) 2021 scientific sessions: virtual congress. The CAPITAL CHILL trial is a prospective single-center randomised, double-blind clinical investigation that was initiated by the investigator. The participants were comatose survivors of out-of-hospital cardiac arrest (OHCA).
A total of 360 patients were to be included in the trial. Any patient over the age of 18 who remained unconscious (Glasgow Coma Score 8) after OHCA and whose treatment plan included TH was included. Patients were allocated to either a goal temperature of 31°C or a goal temperature of 34°C at random. Patients were divided into two groups depending on their initial rhythm at the moment of the OHCA: shockable (ventricular fibrillation or ventricular tachycardia) vs. non-shockable (asystole or pulseless electrical activity). An endovascular device was used to cool all of the patients. The proportion of individuals who die or have a poor neurologic outcome six months after OHCA is the primary outcome. Death, stroke, stent thrombosis, and bleeding are among secondary consequences. An intention to treat analysis was conducted.
The recruitment process began in August 2013 and ended in March 2020. The experiment included a total of 367 participants, and follow-up is now complete. 31ºC Group, n=184. 48.4% of primary outcome was reported in 31ºC group as compared to 45.4% in 34ºC group. 44.45% of mortality was reported in 31ºC group as compared to 41.0% in 34ºC group. 4.9% of poor neurologic outcome was reported in 31ºC group as compared to 4.4% in 34ºC group. Secondary outcomes were 67.4% pneumonia in the 31oC group vs. 63.4% in the 34oC group, 9.2% renal replacement treatment in the 31oC group vs. 9.3% in the 34oC group, 12.5% seizure in the 31oC group vs. 7.1% in the 34oC group, and 4.4% stroke in the 31oC group vs. 1.6% in the 34oC group.
A target temperature of 31 oC did not diminish the incidence of mortality or poor neurologic outcome in comatose survivors of out-of-hospital cardiac arrest at 180 days when compared to a target temperature of 34 oC.

