Effect of Sacubitril/Valsartan on NT-proBNP in Patients with Heart Failure and Preserved Ejection Fraction: The PARAGON-HF Trial
By: Jonathan Cunningham
Key Points
- Sacubitril/Valsartan combination is a neprilysin inhibitor and angiotensin receptor blocker
- PARAGON-HF trial compared Sacubitril/Valsartan vs Valsartan in patients with HF with EF ≥45% and elevated natriuretic peptides with minimum NT-proBNP of >200 pg/ml with HF hospitalization and >300 pg/ml without hospitalization
- 4,796 patients were enrolled for valsartan and Sacubitril/Valsartan run-in period were randomised in 1:1 ratio. The rate of the primary endpoint; Total rate of hospitalization, cardiovascular death was 13% lower with Sacubitril/Valsartan which never met statistical significance. Women and patient with EF benefited more
- The objective of the study was to investigate the relationship between NT-proBNP and outcomes in patients with HFpEF, and the effect of sacubitril/valsartan on NT-proBNP
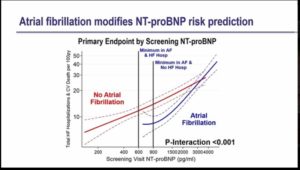
- Baseline NT-proBNP is strongly predicted total HF hospitalization and CV death.
- Patients with atrial fibrillation with low NT-proBNP were excluded from the trial and those with NT-proBNP have high rates of atrial fibrillation. The data suggest the higher minimum NT-proBNP criteria for patients with AF in HF clinical trial
- Non-obese patients showed strong association between NT-proBNP and clinical events and in obese patients this association is weaker
- NT-proBNP strongly predicted events in HFpEF and risk in atrial fibrillation is lower for a given NT-proBNP
- Obese patients with low NT-proBNP retain moderate risk
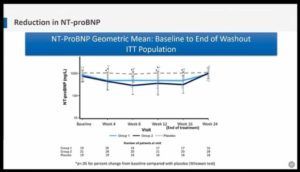
- Sacubitril/valsartan reduced NT-proBNP by 19% vs Valsartan but NT-proBNP showed similar reduction in men/women and lower/higher LVEF
- NT-proBNP did not identify patients who benefit more from Sacubitril/Valsartan
Benefit of Dapagliflozin On First and Repeat Events in Patients with HFrEF in DAPA-HF
By: Piotr Ponikowski
Key Points
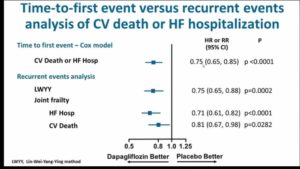
- Among patients with heart failure (HF) and reduced ejection fraction the sodium glucose co-transporter 2 (SGLT2) inhibitor Dapagliflozin reduced the risk of cardiovascular (CV) death or worsening HF in the DAPA-HF trial, in a time-to-first event analysis
- Patient with HF will often experience more than one hospitalization during the course of their disease. Conventional time to first event analysis ignores these repeated hospitalizations
- Also, time-to-first event analysis of a composite outcome such as CV death or HF hospitalization ignores CV death occurring after a first HF hospitalization
- Morever, time-to-first event analyses may overestimate treatment benefit if the treatment effect wanes over time or a subgroup of patients does not respond and has multiple recurrent events
- The objective of the study was to analyse all HF hospitalizations (both first and repeat) experienced by patients in DAPA-HF in this pre-specified secondary analysis
- The primary endpoint was Cardiovascular death or worsening HF event (unplanned HF hospitalization or an urgent HF visit requiring intravenous therapy)
- Patients were randomised to placebo (n = 2371) and Dapagliflozin 10 mg once daily (n = 2373) and follow up was done at day 60, day 120 and then after every 120 days, the follow up continues up to 18.2 months
- Even over a relatively short period of follow-up (median of 18.2 months), a large number of patients experienced recurrent HF hospitalization (32% of all HFH were a second or subsequent event)
- Patients with HF who had recurrent hospitalizations had more advanced disease and more co-morbidities
- Dapagliflozin reduced the risk of both first and recurrent HF hospitalizations. The relative and absolute reductions were large
- The reduction in risk of recurrent HF hospitalizations with Dapagliflozin was more pronounced after accounting for the semi-competing risk of CV death
Contemporary Outcomes with Mitraclip™ (NTR/XTR) System in Primary Mitral Regurgitation: Results from The Global Expand Study
By: D. Scott Lim
Key Points
- The MitraClip system was FDA approved in 2013 for the treatment of subjects with primary (or degenerative) mitral regurgitation who are at prohibitive risk for mitral valve surgery
- Since the original EVEREST II trials, no new core-lab adjudicated data on subjects with primary MR have been reported
- The primary objective of the study was to report on real world, echo core lab and clinical events committee adjudicated outcomes in patients with significant primary mitral regurgitation treated with the next generation MitraClip NTR and XTR systems in the 1000+ patient global EXPAND study
- The secondary objective was to evaluate MR reduction outcomes as a function of mitral valve anatomic complexity and confirm the safety (including single leaflet device attachments and leaflet injuries) and effectiveness of MR reduction associated with the MitraClip NTR and XTR systems
- It was a prospective, multicentre, single arm, international, post-market, real world, observational study conducted in United states, Europe and the Middle east
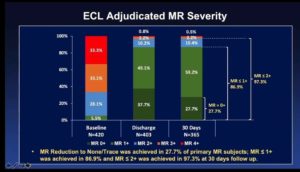
- 1041 subjects consented and underwent MitraClip implantation and 835 subjects enrolled for adequate imaging for ECL assessment in which 422 patients were with primary or mixed etiology and 413 subjects were with secondary MR etiology
- This study represents the first contemporary report of ECL and CEC adjudicated 30 day clinical outcomes in patients with primary MR treated with the next generation NTR and XTR MitraClips
- Approximately one-third of patients had a complex mitral valve anatomy that reflects the difference in patients treated in the real world in comparison to past clinical trials
- MR ≤ 1+ is being achieved more often with MitraClip NTR and XTR than previously observed in EVEREST II trials
- MitraClip XTR was associated with greater MR reduction compared to NTR, in more complex anatomies
Edoxaban Versus Warfarin After Surgical Bioprosthetic Valve Implantation or Valve Repair
By: Geu-Ru Hong
Key Points
- Early postoperative anticoagulation with warfarin is recommended in patients undergoing surgical bioprosthetic valve implantation or valve repair
- However, it is unclear whether direct oral anticoagulant can be an alternative to warfarin in this population
- The objective of the study was to compare the efficacy and safety of edoxaban with warfarin for 3 months in patients who underwent surgical bioprosthetic valve implantation or valve repair
- It was a prospective, randomized, open-labelled, clinical trial conducted between Dec 2017 to Sep 2019 with patients between 20 and 85 years of age
- 285 patients underwent bioprosthetic valve implantation or valve repair and 220 randomised in 1:1 ratio to Edoxaban and warfarin
- Edoxaban group received dose of 60 mg/30 mg orally once daily in patients with 30-5- mL/min creatinine clearance or no more than 60 kg body weight
- In Warfarin group, the dose adjustments were done to maintain the INR between 2.0 and 3.0. The INR measurements were performed daily before discharge and at a scheduled outpatient clinic after discharge
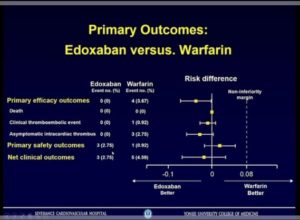
- The primary efficacy outcomes were death, clinical thromboembolic events and asymptomatic intracardiac thrombosis
- The primary safety outcome was occurrence of major bleeding and secondary safety outcome was composite of major or clinically relevant nonmajor (CRNM) bleeding
- Edoxaban is noninferior to warfarin for preventing thromboembolism and occurrence of major bleeding in the first 3 months after surgical bioprosthetic valve implantation or valve repair
- Edoxaban might be an alternative to warfarin in patients early after successful surgical bioprosthetic valve implantation or valve repair
Mavacamten Improves Biomarkers of Myocardial Wall Stress and Injury in Patients with Symptomatic Non-Obstructive Hypertrophic Cardiomyopathy (nHCM): Results from The Phase 2 MAVERICK-HCM Study
By: Carolyn Y. Ho
Key Points
- Hypertrophic cardiomyopathy (HCM) is a primary myocardial disorder. It is unexplained left ventricular (LV) hypertrophy and often caused by pathogenic variants in sarcomeric genes
- Approximately 30% of patients have non-obstructive HCM (LVOT gradient <30 mmHg)
- Cardiac transplantation may be the only option if severe, refractory symptoms were occurred
- By altering the contractile mechanics of the cardiomyocyte, myosin inhibitors have the potential to modify pathophysiology and improve symptoms associated with non-obstructive HCM
- Mavacamten is a first in class, selective allosteric inhibitor of cardiac myosin which reduces the number of myosin-actin cross-bridges and thus decreases excessive contractility characteristic of HCM
- The primary objective of the phase 2, randomised, double-blinded study was the frequency and severity of treatment -emergent adverse events (TEAEs), AEs of special interest and serious adverse events (SAEs)
- Group 1 received Mavacamten ~200 ng/mL and group 2 received Mavacamten ~500 ng/mL and other group received placebo treated for 16 weeks
- Mavacamten was generally tolerated in most participants with non-obstructive HCM and no excess of serious adverse events. LVEF decreased by 4 (8)% in the pooled mavacamten group versus 2.3 (5)% in placebo
- 5 of 40 participants (12.5%) had reversible reductions in LVEF <45% leading to protocol-driven treatment discontinuation
- Treatment with mavacamten resulted in a dose-dependent reduction in serum levels of NT-proBNP, suggesting physiological benefit
- Exploratory analyses suggest that patients with more severe disease expression (baseline elevated cTnl or E/e) may benefit more from mavacamten therapy
- Results set the groundwork for future, larger scale studies in nHCM and potentially in HFpEF
Alirocumab Efficacy and Safety in Adults with Homozygous Familial Hypercholesterolemia (ODYSSEY HoFH)
By: Dirk Blom
Key Points
- HoFH is characterized by extremely high LDL-C levels and early onset atherosclerotic cardiovascular disease despite treatment with conventional lipid lowering therapy
- HoFH includes true homozygotes, compound heterozygotes and double heterozygotes
- HoFH results from severely impaired LDLR function, most commonly due to mutations in both copies of the LDLR gene
- This is the largest randomized controlled interventional trial in HoFH patients to date
- The objective of the randomized, double blind, placebo-controlled, parallel group, phase 3 study was to evaluate LDL-C reduction with the PCSK9 inhibitor alirocumab in adult patients with clinically or genetically diagnosed HoFH
- Patients were randomised in 2:1 ratio to Alirocumab 150 mg (n=45) and placebo (n=24) for 12 weeks in double blind period and Alirocumab 150 mg (n=69) was given in open-label period
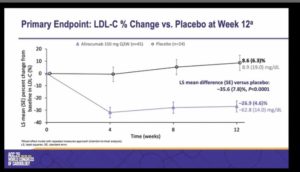
- The primary endpoint was percentage change from baseline in LDL-C vs placebo at week 12
- Alirocumab showed statistically significant and clinically meaningful reduction in LDL-C at week 12 versus placebo
- Consistent reductions in LDL-C were observed from baseline to week 12 for all subgroups, including patients on apheresis
- Alirocumab also significantly reduced ApoB, non-HDL-C, TC and Lp(a)
- LDL-C respose more variable in patients with HoFH than in other forms of hypercholesterolemia
- Alirocumab was generally well tolerated with no distinct safety differences versus placebo
Eicosapentaenoic Acid Levels in REDUCE-IT and Cardiovascular Outcomes
By: Deepak L. Bhatt
Key Points
- In REDUCE IT, 8179 patients were randomised in 1:1 ratio to Icosapent Ethyl (n=4089) and Placebo (n=4090) with continuation of stable statin therapy and follow up for median 4.9 years
- Patients had controlled LDL-C but consistently elevated triglyceride levels despite statin therapy and patients also had established cardiovascular disease or diabetes with at least one risk factor
- The primary endpoint was time to first occurrence of composite CV death, nonfatal MI, nonfatal stroke, coronary revascularisation, unstable angina requiring hospitalization from randomization
- The secondary endpoint was composite of cardiovascular death, MI or stroke
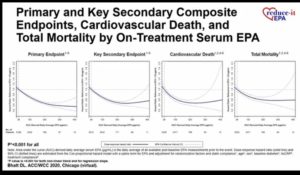
- Icosapent ethyl 4g/day significantly reduced first and total cardiovascular events by 25% and 305 respectively as compared to placebo. These benefits were beyond what can be explained by the degree of triglyceride or other biomarker changes
- On-treatment EPA levels via icosapent ethyl strongly correlate with the primary endpoint
- These data provide a mechanistic underpinning for the large risk reductions seen in multiple endpoints with icosapent ethyl in REDUCE-IT
Natural History of Symptoms and Stress Echo Findings in Patients with Moderate or Severe Ischemia and No Obstructive CAD (INOCA): The NHLBI-funded CIAO Ancillary Study to The ISCHEMIA Trial
By: Harmony R. Reynolds
Key Points
- Ischemia and No Obstructive Coronary Artery Disease (INOCA) shows signs and symptoms of ischemic heart disease with <50% maximal stenosis on coronary angiography and estimated 3-4 million women and men are affected with it
- INOCA mechanisms include:
- Reduced coronary flow reserve
- Epicardial and/or microvascular coronary spasm
- INOCA is associated with increased risk of death, MI, HF and stroke and high healthcare costs similar to patients with CAD
- Persistent symptoms and positive stress testing, eg., stress echocardiography are markers of risk among INOCA patients. However, whether myocardial ischemia is solely responsible for angina in INOCA patients is uncertain
- The objective of the study was to investigate changes in symptoms and stress testing in INOCA patients over 1 year, leveraging the enrolment process of the international, NHLBI-funded ISCHEMIA trial
- Stable patient with moderate or severe ischemia were underwent blinded CCTA. The patients with less CCTA were excluded. As the patients were with no obstructive CAD showed screen failure could have considered trail for if they had ischemic symptoms, enrolled after stress echo
- Angina assessment was done at enrollment, 6 months, 1 year and stress echo repeated at 1 year. Then, comparison between INOCA patients (CIAO) and CAD patients was done
- Longitudinal assessment was done of CIAO patients from baseline to 1 year
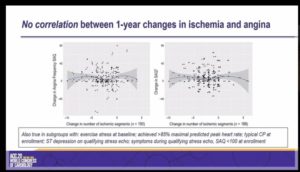
- Primary endpoint was correlation between change in ischemia and change in angina
- CIAO participants were enrolled at 39 sites in 11 countries
- INOCA patients were far more likely to be female, showed largely similar severity of ischemia on stress echo to CAD patients, exhibited more frequent angina but better overall angina related quality of life
- In half of INOCA patients, stress echo is normal at 1 year, 45%same or worse
- Angina frequency improve by a clinically meaningful amount in 39% of INOCA pts, despite little change in anti-anginal medication
- No correlation between change in angina and change in ischemia

