Antidepressant Drugs Should be Avoided if Possible in Epilepsy
Duncan JS, presented a study in a session at 14th World Congress on Controversies in Neurology (CONy): Virtual Congress which assessed whether antidepressant drugs should be avoided if possible in epilepsy. Epilepsy is associated with significantly increased risk of depression and lifetime prevalence is between 6% and 30%. In those with refractory and complex epilepsy who must have bonded to simple therapies attending tertiary centers upto 50% of each individual sometime by significant depression. The highest risk of individuals with focal seizures with impaired awareness in any other terminology these will include complex partial seizure. Suicide risk in individuals with epilepsy is 10 times higher than control risk in large population. It has been reported that some anti-epileptic drugs may make depression worse. In individuals with epilepsy, depression is often unrecognised. The epilepsy should be focus on number of procedures, medications, adherence and symptoms such as tiredness, dizziness, mood and depression may not be sought out hence it is important to actively sought in the patients who are troubled by depression.
In the individuals with depression there is a chance of 4-6 fold increase in the risk of developing epilepsy. Major depression and attempted suicide independently increase risk of subsequent unprovoked seizures. So, depression and epilepsy and significant comorbidity disease and its increased risk is not only due to antidepressant medication or electroconvulsive therapy (ECT). After seizure freedom, depression is most important predictor of impaired quality of life in those with epilepsy (QOLIE-31). Depression has very negative effect on cognition, family, social and financial health. Depression is common in those with epilepsy, it can be severe and it is often unrecognized and needs to treat effectively. Depression is independent risk factor for the development of epilepsy.
Pharmacologic and psychological interventions are essential in the treatment of depression and epilepsy. There are various talking therapies available like cognitive behavioural therapy (CBT), interpersonal therapy, supportive therapy and this can be done in individual, in groups, face to face, and online. Oral antidepressants like tricyclics and bupropion can increase seizure frequency and hence should be avoided in patients with epilepsy. However, the antidepressants like selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs) much less likely to be associated with increased in seizure risk particularly in patients who are already treated with seizures supressing medications. The Danish Head Trauma Database (DHD) showed that SSRI at time of traumatic brain injury (TBI) were associated with increase in risk of developing epilepsy as compared to risk in those individuals who were not prescribed SSRI at the TBI. More likely SSRI are marker for depression and it is underlying course of depression for individual developing epilepsy. Benefits of antidepressant medication in epilepsy are mood, SSRIs reduce susceptibility to seizure induced fatal respiratory arrest in mouse model SUDEP. Adverse effects of antidepressant medication in epilepsy are seizures worse, sedation and dizziness.
Depression is common in epilepsy, is often unrecognised, can be debilitating, having bad effect on quality of life (QoL). But depression can be treated and have markedly effect on QoL so, it is essential need of comprehensive care pathway for depression in patients with epilepsy. Antidepressant medication should not be the first choice and talking therapies can be the first like CBT, interpersonal therapy, supportive therapy. However, if talking therapies are not helpful then modern antidepressant like SSRI have a place in treating depression in epilepsy.
Combination Anti-epileptic Drug Therapy Should be Offered Immediately after Failure of a Single Anti-epileptic Drug
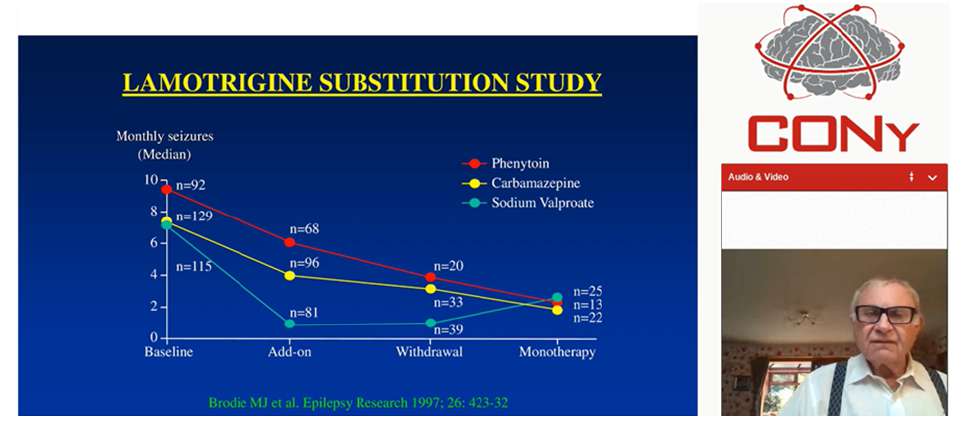
Brodie M, presented a study in a session at 14th World Congress on Controversies in Neurology (CONy): Virtual Congress which analysed whether the combination therapy should be given if single drug fails to show results in epilepsy. In the BASE trial, the number of patients who maintained seizure free were identical with add-on therapy and alternative monotherapy. The higher number of patients remained on add-on treatment as compared to alternative monotherapy. The incidence of adverse effects was lower with add-on therapy (37%) than monotherapy (51%). Also the withdrawal due to side effects was lower with add-on therapy. The lamotrigine substitution study assessed the use of lamotrigine as alternative monotherapy in patients not fully controlled on one established anti-epileptic drug. 129 patients were on Carbamazepine, 92 were on Phenytoin and 117 were on sodium valproate. More patients were seizure free in the add-on therapy than combination therapy. The response rate of patients was higher with combination of lamotrigine and valproate (64%). In a prospective multicentre observational study, 157 males and 174 females aged between 2-86 years were recruited. 239 patients were substituted to alternative monotherapy and 98 patients received add-on treatment. The numbers of patients who remained on alternative monotherapy or add-on therapy were same. The preferred use of alternative monotherapy when the first treatment fails does not necessarily lead to a better outcome in terms of seizure recurrence and adverse effects. Alternative monotherapy should be weighed against duotherapy in terms of expected efficacy, tolerability, ease of use and cost.
In an open cluster randomized, prospective controlled trial 264 patients with persistent focal seizure were evaluated. More patients were seizure free with substitution therapy than combination (51% vs. 45%) and patients on combination therapy showed higher 50% reduction (84% vs. 76%). 232 patients had poor tolerability only and 44% patients of them were controlled on second drug. The problems with substitution were worsening of seizure control on withdrawal of first drug and worsening of side effects when uptitrating second drug unless very slow withdrawal of first drug. The combination drugs should be with different mechanism of action, having no pharmacokinetic interactions, having different side effect profiles and with some supportive evidence in combination.
Combination therapy after failure of the first drug due to lack of efficacy should be offered unless there is also poor tolerability or no benefit at high dosage. The decision to add in another antiseizure drug should also be based on the risk/benefit ratio of substitution or combination relevant to the individual requirements after discussion with patient and family.
New Anti-epileptic Drug are Superior to Old Anti-epileptic Drug
Schulze-Bonhage A, presented a study in a session at 14th World Congress on Controversies in Neurology (CONy): Virtual Congress which evaluate whether the newer anti-epileptic drug (AED) more effective than the established ones. Since 1992, a considerable number of second and third generation AEDs have become available. All underwent clinical trials with class I evidence for improved seizure control when added to the pre-existing AED regime. Despite this, their added value in terms of efficacy has been questioned. One of the reasons for this is; one of the most frequent reported critical sources: repeated case series from one Scottish out-patient clinic failed to show better seizure freedom over time. There is one group where really no improvement in terms of efficacy and this is the treatment of idiopathic generalized epilepsy. So in terms of efficacy, as per the study of standard and new antiepileptic drugs I (SANAD I) (Lamotrigine and Topiramate) and SANAD II (Levetiracetam) trials, it has been reported that valproate is more efficacious than more modern competition. However, valproate is not necessarily the drug of choice in treating generalized epilepsy due to a wide spectrum of shortterm (e.g., tremor) and long term adverse effects (metabolic syndromes with adipositas, polycystic ovary syndrome (PCOS), thrombocytopenia, encephalopathy, osteopathy, teratogenesis, cognitive impairment, etc.) and has been banned for women of childbearing age. It was reported that number of studies suggest similar efficacy between new and old AED.
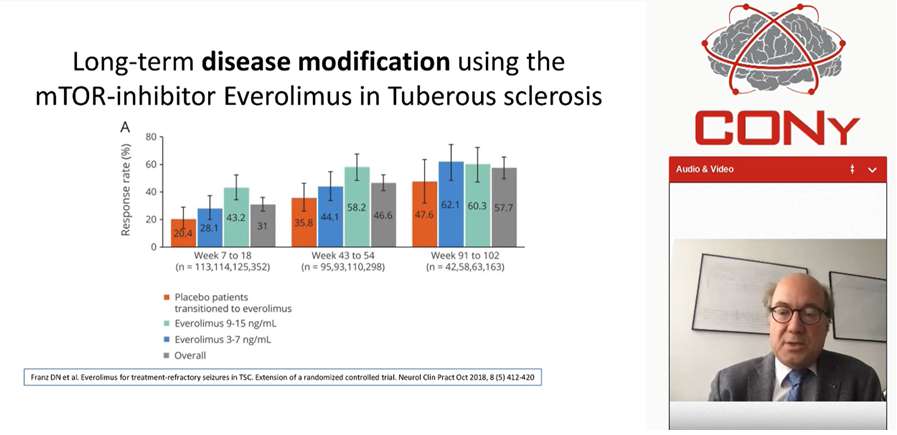
Lamotrigine XR was evaluated in historical control design and showed superior efficacy it was also reported for pregabalin, showed superior efficacy as compared to old AED. While comparing new and old AED the appropriate outcome measure was (a) epilepsy is a chronic condition, (b) for long-term treatment, the separation of efficacy and tolerability is artificial: efficacy in patients not tolerating a drug is irrelevant, and (c) in real life the, the proportion of patients remaining on a drug (efficiency is as an outcome more relevant than isolated short-term effects on seizure frequency). SANAD trial showed superiority of Lamotrigine vs. carbamazepine in a sufficiently powered, long-term comparison of comparison of flexible monotherapy treatments in focal epilepsy. Cenobamate 100 mg, 200 mg and 4000 mg showed 75%, 90% and 100% response rate and complete seizure control in previously pharmacoresisitant focal epilepsy. In case of specific epilepsy syndrome i.e. dravet syndrome, add-on of fenfluramine shows 35% and 75% responders. Similarly, lacosamide showed superiority in brain tumor patients as compared to historical controls and vigabatrin in tuberculosis reported to be prevent epilepsy in this syndrome and certainly first drug i.e., Mammalian target of rapamycin (mTOR) inhibitor everolimus showed long-term disease modification in tuberous sclerosis, this a new agent which not only symptomatically treating epilepsy in tuberous sclerosis but really modifies the disease and gets into mechanism of the disease itself. Some evidences were reported beyond clinical trials: (1) increasingly rare patients with high seizures rates, (2) major reduction of sudden unexpected death in epilepsy (SUDEP) risk with use of modern AED.
There are several lines of evidence supporting superiority of modern 1st AEDs versus 1 generation AED. Valid comparisons require adequate trial designs, including proper powering patient-relevant outcome measures and adequate assessments periods. New AED have proven added and superior efficacy and efficiency both in historical, add-on designs and in long-term monotherapy. Recent studies provide evidence for etiology-specific superiority and possible disease modification for new AED.
Cryptogenic Status Epilepticus Should be Treated with Immunomodulation as soon as it is Diagnosed
Hirsch L, presented a study in a session at 14th World Congress on Controversies in Neurology (CONy): Virtual Congress which analysed whether cryptogenic status epilepticus (SE) should be treated with modulation of the immune system once it is diagnosed. NORSE (New-Onset Refractory Status Epilepticus) is a clinical presentation, not a diagnosis, in patient without active epilepsy other pre-existing relevant neurological disorder, with NORSE without a clear acute or active structural, toxic or metabolic cause. It includes viral infections and autoimmune syndromes. FIRES (Febrile infectionrelated epilepsy syndrome) is a subcategory of NORSE that requires a prior febrile infection, with fever starting between 2 weeks and 24 hr prior to onset of refractory status epilepticus (RSE). Both apply at all ages. In NORSE, 67 from 130 patients (52%) showed cryptogenic SE. 63 patients (48%) had probable etiology. SE leads to increase in interleukin 1 β (IL-1-β), IL-6, tumor necrosis factor α (TNF-α), high mobility group box protein 1 (HMGB1). Early anti-inflammatory treatment in animals improved outcomes and prevented progression and epilepsy. The upregulation of IL-6, IL-8 and other cytokines occurred. NORSE is a genetically determined, postinfectious cytokine-mediated disorder.
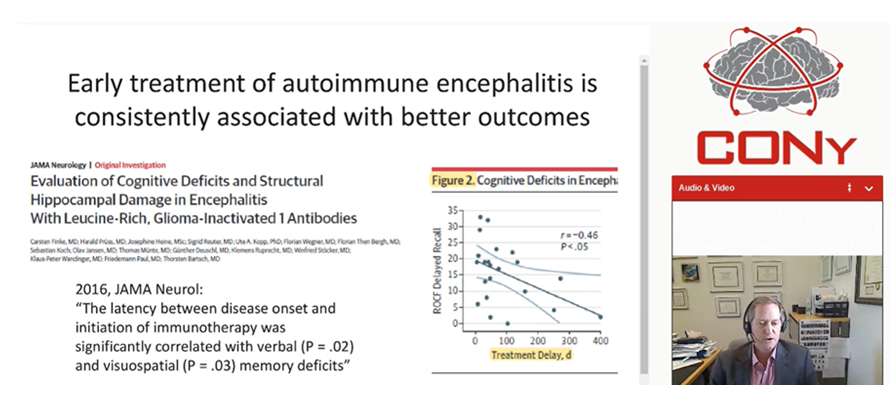
Genetic related to inflammation response may be important and fund differences in an IL-1 related polymorphism associated with risk of FIRES. Anakinra (anti-IL-1) showed efficacy in both acute and chronic phases of pediatric FIRES and in at least one adult with FIRES as well. Tocilizumab showed efficacy in a 6 out 7 adults with NORSE and many had very high IL-6 levels as well. In a study of neuroinflammation in epilepsy, 100 patients were enrolled and multiple cytokines and chemokines showed distinct and more prominent elevation; less in febrile SE and much less in epilepsy and other neurological disorders. Th1-associated cytokines/chemokines [TNF-α, chemokine (C-X-C motif) ligand 9 (CXCL9), CXCL10, CXCL11] as well as IL-6, C-C motif chemokine ligand 2 (CCL2), CCL19 and CXCL1 were elevated. According to Seizure 2013 study, early immunotherapy has been associated with good outcomes in NORSE. In EPIL 2015 study concluded that all patients with RSE to initial treatment in whom an etiology is not immediately apparent, an early administration of immunotherapy should be considered. As per 2016, JAMA Neurol study; 2019, Annal Clin Transl Neurol study; 2013, Lancet Neurol study, early treatment of autoimmune encephalitis is consistently associated with better outcomes. A delay in immunotherapy contributed to a variety of worse outcomes for patients with different subsets of autoimmune encephalitis and delay in commencement of immunotherapy is clearly an important prognostic factor.
Cryptogenic NORSE (incl. FIRES) is very similar if not identical to NORSE of known etiologies. An overactive immune system/ inflammatory response is usually if not always a major contributor to the RSE in NORSE. There is abundant evidence that early immunotherapy is crucial in autoimmune encephalitis, including NORSE.

