Empagliflozin for the treatment of chronic heart failure and a reduced ejection fraction in patients with and without diabetes: New results of the EMPEROR-Reduced trial-Analysis of patients with and without diabetes
Anker S, at the 56th EASD Annual Meeting conducted virtually, presented findings about the new results of EMPEROR-REDUCED trial from a sub group analysis studying patients with and without diabetes. Significant and clinically meaningful benefit was observed in patients with or without diabetes. Diabetes but not prediabetes increases the risk of major heart failure. Empagliflozin reduces the time to first hospitalisation for heart failure (HHF) similarly in patients with and without diabetes.
A substantial reduction of 25-35% of key secondary endpoints i.e totality of hospitalisation for HHF was seen in both groups. Components of primary endpoint were reduced similarly in both the groups, i.e 8% reduction in risk of cardiovascular (CV) death and 30% reduction for HHF. An important aspect of EMPEROR-Reduced trial was also to assess the kidney function. The unconfounded analysis was to reassess patients after the end of treatment for composite renal endpoints. Empagliflozin slowed the rate of decline in renal function over time (unconfounded analysis), and the magnitude of this benefit was not influenced by glycemic status. 50% reduction in the composite renal outcome i.e end stage kidney disease or sustained profound decrease in eGFR was noticed in both groups with Empagliflozin. A similar reduction in metabolic parameters like HbA1c, NT-proBNP, body weight and systolic BP was seen in patients with and without diabetes with Empagliflozin. Empagliflozin did not reduce HbA1c in those without diabetes. No cases of ketoacidosis and no episodes of severe hypoglycaemic episodes were seen in patients without diabetes.
Conclusion: Patients with HFrEF and diabetes are at higher risk of heart failure and renal events – the increased risks is not seen in patients with pre-diabetes. Empagliflozin reduced the risk of HHF and slowed the rate of decline in renal function similarly with or without diabetes. Decisions regarding the use of empagliflozin for the treatment of heart failure and reduced ejection fraction should not be influenced by the glycemic status of individual patients.

The important conversation between the heart and the kidney: Pharmacological approach to cardiorenal disease in type 2 diabetes
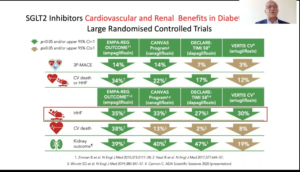
Zannad F, at the 56th EASD Annual Meeting conducted virtually, presented a session on pharmacological approach to cardiorenal disease in type 2 diabetes (T2D). The cardiovascular and renal system are affected by common pathways that lead to systemic changes and organ dysfunction. Diabetes and hypertension both cause sympathetic neurohormonal activation, inflammation, endothelial dysfunction, fibrosis, oxidative stress ultimately leading to organ damage. RAAS activation and other profibrotic pathways cause fibrosis in both kidneys and the heart. Medications investigated concomitantly for CV and kidney benefits in T2D include the RAAS inhibitors, ARNis (sacubitril, valsartan), SGLT-2 inhibitors and GLP-1 receptor agonists. DPP-4 inhibitors have been proven ineffective in preventing both renal and cardiac outcomes. GLP-receptor agonists have shown heterogeneous results in CV and renal outcomes. Majority of the landmark trials failed to show consistent reductions in CV and renal outcomes except for SUSTAIN and LEADER trials which exhibited significant reduction in both CV and renal outcomes. Also, GLP-1 receptor agonists failed to show any benefit on HF outcomes-an important CV parameter. Trials evaluating SGLT-2 inhibitors like EMPA-REG, CANVAS, DECLARE-TIMI and VERTIS-CV have shown overall consistent effect in reducing CV and renal outcomes with the greatest effect in reducing HHF. The CREDENCE trial also showed significant reduction in risk of primary composite kidney outcome and other CV outcomes in patients with T2D and albuminuric CKD. There was 30% relative risk reduction in end stage kidney disease, doubling of serum creatinine and death from kidney causes or CV death. Finerenone a nonsteroidal anti-mineralocorticoid is being studied in clinical trials like FIDELIO and FIGARO for the treatment of chronic kidney disease in people with T2D. In the EMPEROR-Reduced trial, consistent benefit was seen in kidney and cardiac endpoints with empagliflozin.
Conclusion: In the future, a harvest of trials will be demonstrating results in cardiorenal patients. Certainly, the role of RAAS inhibition is of utmost importance in alleviating kidney and cardiac outcomes. In addition, the role of SGLT-2 inhibitors beyond diabetes management needs to be acknowledged.
DAPA-CKD trial: Results of the DAPA-CKD trial
Heerspink HL, presented a new research at 56th EASD Annual Meeting conducted virtually, which proves that dapagliflozin, primarily used to treat type 2 diabetes, reduces the risk of declining kidney function, kidney failure, heart failure and death in patients with chronic kidney disease (CKD), irrespective of their diabetes status. 4304 participants with an eGFR of 25-75 ml/minute/1.73m2 (stages II-IV CKD) and urinary albumin-to-creatinine ratio 200-5000 mg/g were randomised to dapagliflozin (10 mg once daily) or placebo. The primary endpoint was a composite of sustained decline in estimated GFR of at least 50%, end-stage kidney disease, or death due to kidney-related or cardiovascular causes. It was observed that over a median 2.4 years, the primary outcome occurred in 9.2% in the dapagliflozin group and 14.5% in the placebo group, signifying patients in the dapagliflozin group had a 39% lower risk of the primary endpoint compared with the placebo group. In a series of secondary analyses, patients in the dapagliflozin group also had a 44% lower risk of the combined outcome of sustained decline in eGFR of ³ 50%, end-stage kidney disease, or death from only kidney causes compared with placebo group. Similar to the results of DAPA-HF study, dapagliflozin in DAPA-CKD study showed 29% lower risk of the combined endpoint of either cardiovascular death or hospitalisation for heart failure Neither diabetic ketoacidosis nor severe hypoglycaemia were observed in patients without type 2 diabetes.
Conclusion: DAPA-CKD trial proved that patients with CKD who received dapagliflozin as a treatment demonstrated a significantly lower risk of experiencing kidney failure, death from cardiovascular causes or hospitalisation for heart failure, and had a prolonged survival compared with those who received placebo, irrespective of the presence of type 2 diabetes. The marked reduction in mortality supports the use of dapagliflozin as a vital addition to the armamentarium of the management of patients with chronic kidney disease.

Do we need surveillance of the liver of type 2 diabetes patients?
Trauner M, presented a session at the 56th EASD Annual Meeting conducted virtually, which discussed about the need of surveillance of the liver in patients with type 2 diabetes. The authors addressed 3 key questions: why we should care, how we should care and what are the clinical consequences and action (if any) surveillance of the liver in patients with type 2 diabetes? Liver diseases in T2DM, mainly non-alcoholic fatty liver disease (NAFLD), chronic hepatitis C and Acute liver failure causes 4.4-12.5% deaths in T2DM. NAFLD is hepatic manifestation of metabolic syndrome showing metabolic co-morbidities; not only diabetes and obesity but also associated CV risk, renal disease which is receiving tremendous attention. In NAFLD, about 35% patients may develop hepatocellular carcinoma at pre-cirrhotic stage. NAFLD is referred as a neglected complication of T2DM. Lifestyle, genetic factors, metabolism, microbiota are main drivers of NAFLD/NASH. The extrahepatic manifestation of NAFLD is chronic kidney disease, which shows much higher prevalence in NASH patients than general population. For NAFLD screening, ADA 2020 guidelines recommends that patients with type 2 diabetes or prediabetes and elevated liver enzymes or fatty liver on ultrasound should be evaluated for presence of NASH and liver fibrosis. Normal ALT levels do not rule out advance fibrosis and cirrhosis due to NASH. Fibrosis is the major determinant of mortality in NAFLD and risk of mortality increases with fibrosis stage in NAFLD. Available non-invasive tests with two different but complementary approaches are biological approach and physical approach. The diagnosis of cirrhosis i.e. advanced chronic liver disease is done through endoscopy which shows low risk varices and high risk varices. Low risk varices are treated with beta-blockers while high risk varices are given primary prophylaxis. The risk of hepatocellular carcinoma (HCC) increases with diabetes duration in patients with T2D. Current guideline treatment recommendations for NASH patients are diet, exercise and managing co-morbidities. However, for NASH patients with advanced fibrosis, new treatments are urgently needed because they are at greatest risk of progression to cirrhosis and other serious conditions. No drugs are approved for NASH and no specific therapy can be recommended.
Conclusion: It is important to undertake liver surveillance to evaluate prognostic implications for hepatic outcomes and future implications for cardiometabolic outcomes. Care can be ensured through ordering for non-invasive tests for liver fibrosis and clinically practicable algorithms. Clinical consequences and action may include surveillance for HCC and portal hypertension and combining cardiometabolic with antifibrotic therapies.
Functional changes in beta cells during ageing and senescence
Aguayo-Mazzucato C, presented a session at the 56th EASD Annual Meeting conducted virtually which explored the functional changes in beta cells during ageing and senescence. Type 2 diabetes is an age-related disease. Diabetes is characterised by decrease in β-cell mass and dysfunctional insulin secretion. Age decreases proliferative capacity of β-cells, which seems to be mediated by p16Ink4a. β-cell mass in human reported not to change or decrease with ageing. Ageing decreases the ability of β-cells to proliferate in rodents and humans which is highly correlated with β-cell senescence. Many studies demonstrate the relation between age and β-cell function in which at young age from 1 month upto 20 months increased basal insulin secretion is observed and from age 20 to older age more than 60 years, the decrease in β-cell function is seen. In a study, Islets from older animals showed secretion of β-cell characterised by increased basal insulin secretion and this renders them less responsive to high glucose challenge. β-cell functional decline correlates to progressive insulin resistance. The 9 hallmarks of ageing are genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, stem cell exhaustion, cellular senescence and senescence associated secretory phenotypes. The latter two are enhanced in pathway analysis of microarray data from purified β-cells from 1 and 2-year-old mice. Generation of β-cell senescence signature reveals loss of cellular identity. Senescence and chronological ageing produces similar changes in β-cell gene expression. Deletion of p16Ink4a expressing cells improved β-cell function. Senotherapeutics target preferentially senescent cells and spare non-senescent cells. Senomorphic treatment effectively decreases basal insulin secretion from human islets.
Conclusion: β-cell function declines with age. This is characterized by an increase in basal insulin secretion. Cellular senescence is one of mechanisms behind these functional changes. Targeting senescent β-cells through senotherapeutics, restores basal insulin secretion. Inhibition of SASP, through senomorphics seems to be the main mechanism through which functional restoration occurs.
Cardiovascular (CV) and hypoglycaemia outcomes across age groups in people with type 2 diabetes in the CAROLINA trial
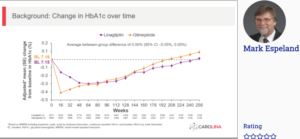
Espeland MA, at the 56th EASD Annual Meeting conducted virtually, presented findings from the CAROLINA trial which analysed the impact of Linagliptin 5 mg versus Glimepiride 1-4 mg once daily on cardiovascular (CV) and hypoglycaemia outcomes across age groups. Adults with relatively early T2D, HbA1c 6.5-8.5%, and increased CV risk were recruited in the CAROLINA trial. The primary outcome was CV death, non-fatal myocardial infarction, or non-fatal stroke (3P-MACE); secondary outcomes were mortality and changes from baseline in HbA1c, weight and hypoglycaemia.
846 (14.0%) patients were ≥75 years from 6033 patients (median age 64 [range 36-85]) years. CV and mortality effect rates did not vary among therapy groups, altogether and over age classes (interaction p-values >0.05) in median follow-up of 6.3 years. No meaningful difference was observed in HbA1c among Linagliptin vs glimepiride following some initial differences, altogether and over age groups. Significantly lower risk for hypoglycaemia with linagliptin, as well as modest weight loss, with no difference across age groups was observed. CV and mortality outcomes were similar with linagliptin or glimepiride across age groups at high risk for CV events. Fewer falls or bone fractures with linagliptin than with glimepiride, with consistent risk reduction in bone fracture across age groups and reduced risk of falls in participants aged ≥ 75 years. Collectively, these are important safety considerations when selecting therapy for the elderly.
Conclusion: Results for CV and mortality outcomes were compatible over age subgroups, however Linagliptin showed modest advantage in weight and a substantially lower hypoglycaemia burden as compared to Glimepiride, which are significant safety factors when selecting treatment for the elderly.
Reduction of cardiovascular events by GLP-1 receptor agonists is explained by HbA1c reduction
Roosimaa M, presented a meta-analysis report at the 56th EASD Annual Meeting conducted virtually to assess whether better glycemic control can explain decreased CV risk seen with glucagon-like peptide-1 receptor agonists (GLP-1RA). Multiple cardiovascular outcome trials (CVOT) with sodium-glucose transport protein 2 inhibitors (SGLT2i) and GLP-1RA have provided an unexpected reduction in CV risk ever since the FDA has issued guidance for the assessment of cardiovascular (CV) safety of new antidiabetic medications. Despite trials like DCCT and UKPDS with better glycemic control resulting in a lower rate of CV complications after prolonged follow-up, this finding has not been confirmed in ADVANCE, ACCORD and VADT trials where older patients with already established CV risk factors, a population more similar to patients in current CVOTs, was included. Hence, the importance of glycemic control as a mediator of risk reduction in CVOTs has been questioned.
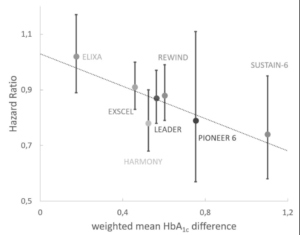
In the study the absolute risk of primary outcome, HbA1c and number of patients at risk was extracted for both GLP-1RA and placebo groups at multiple time points from each published GLP-1RA trial like ELIXA, LEADER, SUSTAIN-6, PIONEER 6, Harmony Outcomes, EXSCEL, REWIND.
The relationship between risk and glycemic control was assessed by two methods: 1) Cumulative glycemic exposure was calculated separately for placebo and GLP-1RA groups for each trial and correlated with observed absolute CV risk. 2) Differential glycemic exposure between GLP-1RA and placebo group was calculated for each trial and correlated with a published hazard ratio (HR). A clear linear correlation was established between increasing HbA1c exposure and CV risk both with GLP-1RA and placebo. Placebo group appeared to have a lower absolute risk with the same glycemic exposure, but this difference was lost after correction for patient age. The difference in glycemic exposure between GLP-1RA and placebo group had a strong correlation (R =0,75) with reported hazard ratio and 95% confidence intervals of HR-s from all trials included risk reduction expected from HbA1c difference. Lowering of HbA1c by 1% decreased CV risk by 30% and this relationship was consistent within placebo groups, GLP-1RA groups and between groups.
Conclusion: The reduction of CV events in CVOTs with GLP-1RA can be explained by a reduction in HbA1c.
Exploring potential mediators of the cardiovascular benefit of dulaglutide in REWIND
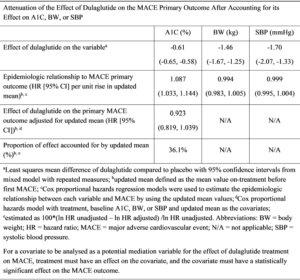
Gernstein H, presented a post-hoc assessment report at the 56th EASD Annual Meeting conducted virtually which estimated the degree to which the effect of dulaglutide (DU) on risk factors like HbA1c, body weight (BW), and systolic blood pressure (SBP) could statistically account for its effect on major adverse cardiovascular event (MACE-3). The REWIND trial showed that relative to placebo (PL), once weekly DU 1.5 mg reduced the incidence of a MACE-3; nonfatal myocardial infarction, nonfatal stroke, or cardiovascular (CV) death) in patients with type 2 diabetes with and without established CV disease (hazard ratio [HR] 0.88, 95% CI [0.79, 0.99]; p=0.026). DU also significantly reduced HbA1c, body weight (BW), and systolic blood pressure (SBP).
Data were analysed from 9,901 patients who had 1,257 first MACE-3 over 5.4 median years of observation. Those risk factors for which the updated mean on follow-up was significantly related to cardiovascular events were added to a separate Cox model that included DU allocation, the baseline value of the measurement and the updated mean of the variable as time dependent covariates. The results observed were only HbA1c meeting this condition, suggesting that BW and SBP did not mediate the effect of DU on MACE in this study population. The effect size of DU on the MACE outcome was attenuated by 36.1% after accounting for its effect on HbA1c.
Conclusion: The results suggest most of the CV benefits of dulaglutide on MACE are not attributable to the HbA1c, BW, or SBP-lowering effects of dulaglutide.
Linagliptin as add-on treatment to glimepiride in patients with HNF1A-diabetes (MODY3): a randomised, double-blinded, placebo controlled, crossover trial
Christensen AS, presented a study report at the 56th EASD Annual Meeting conducted virtually which hypothesised that a low dose of sulfonylurea in combination with the dipeptidyl peptidase 4 inhibitor linagliptin provides a more efficacious treatment with less glycaemic variability and hypoglycaemia in patients with hepatocyte nuclear factor 1- alpha HNF1A-diabetes (MODY3)-diabetes compared to sulfonylurea monotherapy.
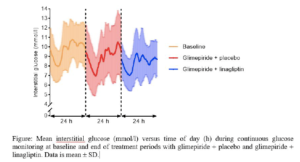
This was a randomised, double-blinded, cross-over trial, where patients with HNF1A-diabetes (n=20, [mean ± SD]; age: 43 ± 14 years; BMI 24.3 ± 2.8 kg/m2; HbA1c 57.1 ± 7.3 mmol/mol) were randomly assigned to treatment with glimepiride + linagliptin 5mg (16 weeks), wash-out (4 weeks) and treatment with glimepiride + placebo (16 weeks). Glimepiride was titrated in a treat-to-target manner aiming for fasting plasma glucose of 4.5-6.0 mmol/l without hypoglycaemia. Treatments were evaluated by continuous glucose monitoring (CGM), HbA1c and a mixed-meal test at baseline and at the end of each treatment period. The primary endpoint was difference between treatments in mean amplitude of glycaemic excursions (MAGE) and secondary endpoints included differences between treatments in coefficient of variation (CV) on CGM, HbA1c and hypoglycaemia. The results observed were: Glimepiride dose was significantly reduced ([mean difference, 95% CI] -0.7 [1.2 to 0.2] mg/day) with glimepiride + linagliptin compared with glimepiride + placebo. Glimepiride + linagliptin when compared to glimepiride + placebo, showed an incognisant reduction in MAGE (-0.7 [-1.9 to 0.4] mmol/l) and significant improvements in CV (-3.7 [-7.1 to -0.3] %), HbA1c (-5.6 [-9.3 to -1.8] mmol/mol), body weight (-1.0 [-2.0 to -0.1] kg) and beta cell function. Incidence of hypoglycaemia was similar in the two treatment periods.
Conclusion: Linagliptin as add-on treatment to glimepiride in patients with HNF1A-diabetes improved glycaemic variability control and reduced the daily glimepiride dose without increasing the risk of hypoglycaemia.
Nasal glucagon was efficacious in reversing insulin-induced hypoglycaemia without increasing risk of secondary hyperglycaemia
Giménez M, presented a study report at the 56th EASD Annual Meeting conducted virtually to evaluate efficacy, pharmacodynamics, and safety of nasal glucagon compared to injectable glucagon in reversing insulin-induced hypoglycaemia in Caucasian and Japanese adults with type 1 diabetes (T1D) or type 2 diabetes (T2D). This post hoc analysis used data from 2 randomized, cross-over studies. Treatment success was defined as an increase in blood glucose to ≥3.9 mmol/L or an increase of ≥1.1 mmol/L from nadir blood glucose within 15 minutes of receiving glucagon. Blood glucose was measured every 5 minutes for the first 30 minutes, every 10 minutes up to 60 minutes, and at varied extended time intervals thereafter. Pharmacodynamics data, including area under the curve above 7.8 mmol/L (Δ7.8 AUC [1-4 hr]), were used to evaluate the risk of secondary hyperglycaemia. Tolerability was assessed with treatment-emergent adverse events and a nasal symptom questionnaire.
The results observed a similar proportion of nasal glucagon (97.8% [131/134)] and injectable glucagon patients (97.0% [130/134]) achieved treatment success. Mean time to treatment success (for blood glucose increase) was 11.7 minutes for nasal glucagon and 10.4 minutes for injectable glucagon (p<0.001). The median time for both nasal and injectable glucagon was 10 minutes. Geometric least square mean maximal blood glucose (BG) for nasal glucagon and injectable glucagon were 10.8 and 11.4 mmol/L (p<0.001), respectively. Nasal glucagon had significantly lower Δ7.8 AUC (1-4 hr) (p<0.001), with 42% reduction compared to injectable glucagon. Nasal glucagon had similar rates of nausea (19.1%) and vomiting (8.5%) versus injectable glucagon (28.8% and 11.5%, respectively), with higher rates of side effects related to nasal administration (headache [7.8% nasal glucagon, 5.8% injectable glucagon], upper respiratory tract irritation [6.4% nasal glucagon, 0.7% injectable glucagon]). Separate T1D and T2D analyses showed similar results as the T1D+T2D groups combined.
Conclusion: Nasal glucagon was efficacious and well tolerated in reversing insulin-induced hypoglycaemia in adults with T1D or T2D and did not increase the risk of secondary hyperglycaemia compared to injectable glucagon.
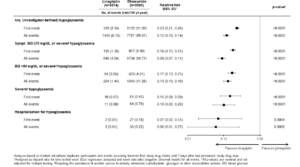
Incident and recurrent hypoglycaemia with linagliptin and glimepiride in the CAROLINA trial
Zinman B, presented a study at the 56th EASD Annual Meeting conducted virtually to report the effects of treatment on a range of hypoglycaemia outcomes in the CARdiovascular Outcome Study of LINAgliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA). The CAROLINA trial recruited adults with relatively early T2D, HbA1c 6.5-8.5%, and elevated CV risk who were randomized to linagliptin 5 mg or glimepiride 1-4 mg once daily added to usual care. Time to first and sum of first plus recurrent hypoglycaemia were assessed using proportional hazards and negative binomial regression across the following categories: any; symptomatic with blood glucose (BG) ≤70 mg/dL or severe; BG <54 mg/dL or severe; severe; and leading to hospitalisation. 6033 participants (mean age 64.0 years, HbA1c 7.2%, median T2D duration 6.3 years, 42% with CV disease) were followed for median 6.3 years.
The results observed were HbA1c over time did not differ between groups, but a significantly lower frequency of hypoglycaemia events was observed with linagliptin (Fig), regardless of definition, a difference further accentuated when including recurrent hypoglycaemia.
Conclusion: When compared with glimepiride, linagliptin demonstrated significantly lower hypoglycaemia burden. Provided the potentially harmful clinical impact of hypoglycaemia, these data may help inform therapy selection.