Learning from DAPA-HF one year on: Expert on the Spot Exploring new learnings from DAPA-HF
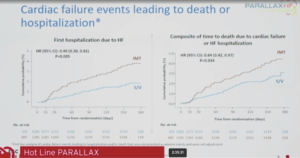
McMurray presented the new learnings from DAPA-HF at the ESC Congress 2020: A digital experience. The key results of the two SGLT2i trials in heart failure – DAPA-HF and EMPEROR Reduced showed that the composite outcome i.e. CV death or HF hospitalization which is primary endpoint in EMPEROR-Reduced and secondary endpoint in DAPA-HF, their results are almost identical. The HR ratio or RR ratio of the heart failure hospitalization occurs first hospitalization and total hospitalization are identical in DAPA-HF and EMPEROR Reduced trials. However, in cardiovascular death and all-cause death, there is a difference between the HR ratio of this two trials. The other outcomes include KCCQ-CSS change from baseline, there is an increase in score which corresponds with improvement symptoms or quality of life or health status. The composite renal endpoint result was quite differently in two trials. The no. of events was small in both trials and is numerically less with SGLT2i compared to placebo in both trials. In eGFR, both SGLT2i slowed the rate of decline in eGFR over time. In DAPA-HF, NT-proBNP tend to rise, in placebo group, NT-proBNP consistent worsening with time but in Dapagliflozin group there was a reduction in NT-proBNP. Empagliflozin and Dapagliflozin are structurally similar and both tested in large trials and both being shown reduction in mortality. In DAPA-HF, the event rates by LVEF category was lower in Dapagliflozin group as compared to placebo group; the treatment effects by continuous LVEF, consistent reduction was seen in cardiovascular death across the spectrum of EF in DAPA compared to placebo. In treatment effect according to NT-proBNP, the continuous HR ratio is consistently below unity 1, it can be questioned that the benefit may be even greater in people with lower NT-proBNP level, that can be more interesting to see in EMPEROR Reduced trial.
Conclusion: SGLT2i reduce the risk of HF hospitalization and CV death. SGLT2i improve/prevent worsening of symptoms. SGLT2i slow deterioration in kidney function over time. Crucially these benefits are obtained on top of conventional therapy, including Sacubitril/valsartan.
Learning from DAPA-HF one year on: Expert on the Spot Contextualising new learnings from DAPA-HF – A patient case-led discussion
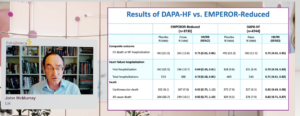
Boer presented a session on ontextualising new learnings from DAPA-HF – A patient case-led discussion at the ESC Congress 2020: A digital experience. A case study of Mr Khan, a 65-year-old man, a patient of heart failure, starting with symptoms, physical examination is not very clearly known. Heart rate is 83 bpm. Very importantly, in atrial fibrillation the BP is fortunately not too low 130/80 mmHg. A typical risk befitting HF history; hypertension, type 2 diabetes, comorbidities including COPD, ankle edema. On an ECG, there is biventricular enlargement and EF is 26%. Before going to treatment some haematology with creatinine 123 mmol/L, eGFR 52 mL/min/1.73m2, is quite typical with patient with HFrEF and history of T2DM. The HF patient will treat with losartan, furosemide and also to manage hypertension he is taking Amlodipine, for atrial fibrillation he is using Doxepin, and further disease management is known with DPP4i, gliptin, metformin and further he is using LABA and LAMA to manage his COPD. The question is would this patient is eligible for a SGLT2i. A DPP4i is a drug that lowers glycated haemoglobin but interestingly we have better study with gliptin in patients with HFrEF and type 2 diabetes. In fact, gliptin is associated with increase in ventricular volume, so DPP4i wouldn’t be given as a glucose lowering therapy in patients with HF. The glucose lowering therapy would be a SGLT2i and its using in patients because it is also a treatment for HFrEF so, SGLT2i would be two treatments for one particular man. If this patient is diagnosed with systolic failure, first step would be to install guideline recommended treatment, Losartan can be acceptable, the dose should be 100 mg. According to guidelines, from post hoc analysis, efficacy of beta-blockers for patients with HFrEF and atrial fibrillation is slightly less than for HF patients in sinus rhythm so, rate control is acceptable.
Conclusion: In coming future, we should probably come up with a key schemes much more manageable for doctors and patients where we start right away with more modern therapeutic modalities.
DAPA-CKD – Dapagliflozin in Patients with Chronic Kidney Disease
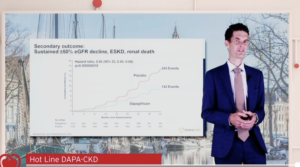
The findings of the DAPA-CKD trial at the ESC Congress 2020: A digital experience was presented by Professor Heerspink. The DAPA-CKD trial tested the hypothesis that treatment with dapagliflozin is superior to placebo in reducing the risk of renal and cardiovascular events in patients with chronic kidney disease (with or without type 2 diabetes) already receiving a stable dose of either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) as background therapy. The primary composite endpoint was worsening kidney function (defined as >50% sustained decline in estimated glomerular filtration rate [eGFR] or onset of end-stage kidney disease), or death due to kidney disease or cardiovascular disease. The trial enrolled 4,304 patients, aged 18 years and over, from 386 centres in 21 countries. All patients had an eGFR ≥25 and ≤75 mL/min/1.73m2; urinary albumin to creatinine ratio between ≥200 mg/g and ≤5000 mg/g; and were on a stable, maximum tolerated dose of an ACE inhibitor or ARB (unless contraindicated) for at least four weeks. Patients were randomly allocated to dapagliflozin 10 mg or placebo once daily in addition to standard of care (i.e. an ACE inhibitor or ARB). The mean age of participants was 61.8 years; 66.9% were male and 67.5% patients had type 2 diabetes. During a median follow-up of 2.4 years, there were 197 primary endpoint events with dapagliflozin and 312 with placebo. The HR for the primary endpoint was 0.61 (95% confidence interval [CI) 0.51–0.72; p=0.000000028). Consistent benefit of dapagliflozin on the primary endpoint was noticed in patients with and without type 2 diabetes. Dapagliflozin reduced all three secondary endpoints compared to placebo. The HRs were: 1) worsening renal function or death from kidney failure 0.56 (95% CI 0.45–0.68; p<0.0001); 2) hospitalisation for heart failure or cardiovascular death 0.71 (95% CI 0.55–0.92; p=0.0089); and 3) all-cause mortality 0.69 (95% CI 0.53–0.88; p=0.0035).
Conclusion: In DAPA-CKD trial, Dapagliflozin reduced the risk of worsening kidney function or death from cardiovascular or kidney disease in patients with chronic kidney disease with and without type 2 diabetes.
2020 ESC guidelines on non-ST-segment elevation acute coronary syndromes
Five years since the last version of the guidelines, the 2020 ESC guidelines on NSTE-ACS was presented today at the ESC Congress 2020: A digital experience. Several new key recommendations were presented. Some of the key new recommendations include:
- For diagnosis with rapid ‘rule-in’ and ‘rule-out’ algorithms, it is now recommended to use the ESC 0 h/1 h algorithm (best option, blood draw at 0 h and 1 h) or the ESC 0 h/2 h algorithm (second-best option, blood draw at 0 h and 2 h) if a high-sensitivity cardiac troponin (hs-cTn) test with a validated algorithm is available
- If elective non-invasive or invasive imaging is needed after the rule-out of myocardial infarction (MI), invasive coronary angiography is deemed to be the best option in patients with very high clinical likelihood of unstable angina
- Nevertheless, stress testing with imaging or coronary computed tomography angiography (CCTA) is seen as the best option in patients with low-to-modest clinical risk
- Rhythm monitoring up to 24 hr or to PCI (whichever comes first) is recommended in patients with NSTE-ACS at low risk for cardiac arrhythmias, while monitoring for >24 h is recommended in patients at increased risk for cardiac arrhythmias with Class I recommendation
- In patients considered at low risk, a selective invasive strategy after appropriate ischaemia testing or detection of obstructive coronary artery disease by CCTA is recommended
- Routine administration of P2Y12 receptor inhibitor pre-treatment in NSTE-ACS patients is not recommended in whom coronary anatomy is not known and an early invasive management is planned, given the lack of established benefit, although it may be considered in selected cases and according to bleeding risk
- The new recommendation in patients with atrial fibrillation and an indication for oral anticoagulation, is a dual antithrombotic strategy after a short period of triple antithrombotic therapy (<7 days)
- For those patients at high ischaemic risk, triple antithrombotic therapy may be extended up to 4 weeks
- A set of quality indicators are aligned with the current recommendations to take into account the wider NSTE-ACS pathway of care

Dapagliflozin reduces the risk of hyperkalaemia in patients with heart failure and reduced ejection fraction: A secondary analysis DAPA-HF
Hyperkalaemia frequently restricts the use of mineralocorticoid receptor antagonists (MRAs) in patients with heart failure and reduced ejection fraction (HFrEF), refusing these patients a life-saving treatment. Kristensen SL, presented an ePoster at the ESC Congress 2020-: A digital experience, which analysed whether sodium-glucose cotransporter 2 (SGLT-2) inhibitor Dapagliflozin treatment decreases the risk of hyperkalaemia correlated with MRA use in patients with HFrEF.
The risk of arising mild hyperkalaemia (potassium > 5.5 mmol/L) and moderate/severe hyperkalaemia (>6.0 mmol/L) was analysed in the Dapagliflozin And Prevention of Adverse-outcomes in Heart Failure trial (DAPA-HF) as per the background MRA use, and randomized therapy allocation, by Cox regression analyses. Overall, 3370 (70.1%) patients were managed with an MRA in DAPA-HF. Mild hyperkalaemia and moderate/severe hyperkalaemia arised in 182 (11.1%) and 23 (1.4%) patients treated with Dapagliflozin as compared to 204 (12.6%) and 40 (2.4%) of patients given placebo (Table and Figure). The mild hyperkalaemia showed a hazard ratio (HR) of 0.86 (0.70-1.05) and 0.50 (0.29, 0.85) for moderate/severe hyperkalaemia, comparing Dapagliflozin to placebo.
Conclusion: Patients with HFrEF and taking a MRA who were randomized to Dapagliflozin showed half the occurence of moderate/severe hyperkalaemia, as compared to those randomized to placebo.
|
|
Dapagliflozin |
Placebo |
|
|
|||
|
|
No. events/patients |
Rate per 100py
|
No. events/patients |
Rate per 100py |
HR (95% CI) |
p-value |
|
|
Mild hyperkalaemia (>5.5 mmol/L)* |
|
|
|
|
|
|
|
|
No MRA at baseline |
63/661 |
7.1 |
58/684 |
6.5 |
1.20 (0.84-1.72) |
0.32 |
|
|
MRA treated at baseline |
182/1637 |
8.6 |
204/1626 |
9.8 |
0.86 (0.70-1.05) |
0.14 |
|
|
All patients |
245/2298 |
8.2 |
262/2310 |
8.8 |
0.93 (0.78-1.11) |
0.42 |
|
|
Moderate/Severe hyperkalaemia (>6.0 mmol/L)** |
|
|
|
|
|
|
|
|
No MRA at baseline |
13/676 |
1.4 |
11/697 |
1.1 |
1.17 (0.52-2.62) |
0.71 |
|
|
MRA treated at baseline |
23/1688 |
1.0 |
40/1667 |
1.7 |
0.50 (0.29-0.85) |
0.010 |
|
|
All patients |
36/2364 |
1.1 |
51/2364 |
1.6 |
0.64 (0.42-0.99) |
0.046 |
|

The effect of dapagliflozin in patients with HFrEF and COPD: a post-hoc analysis of DAPA-HF
Chronic obstructive pulmonary disease (COPD) is a significant comorbidity in HFrEF, correlated with worse effects and suboptimal therapy because of under-prescription of beta-blockers. Therefore, additional effective treatments are particularly appropriate in patients with COPD. In DAPA-HF, the sodium-glucose cotransporter 2 (SGLT-2) inhibitor, Dapagliflozin decreased risk of cardiovascular (CV) death or worsening heart failure (HF) in patients with HF with reduced ejection fraction (HFrEF) compared to placebo. Thus, Dewan P, presented an ePoster at the ESC Congress 2020: A digital experience which analysed outcome of Dapagliflozin in patients with and without COPD compared with placebo.
Primary composite outcome of DAPA-HF was time-to-first CV death or worsening HF occurrence (hospitalization for HF or outpatient visit requiring intravenous therapy). It was analysed that whether effect of Dapagliflozin was modulated by investigator reported COPD at baseline. Overall, 585 (12.3%) of the 4744 randomized patients showed an investigator-reported history of COPD, 299 (12.6%) in Dapagliflozin group and 286 (12.1%) in placebo group. Placebo group showed higher incidence of primary composite outcome in patients with COPD than in those without (22.8; 95% CI 18.4-28.3 vs. 14.9; 13.5-16.4) (Table). The effect of Dapagliflozin showed constant hazard ratio (HR) on the primary outcome as compared to placebo, in patients with and without COPD (Table); p-value for interaction was 0.467. Findings for other outcomes were identical.
Conclusion: Patients in DAPA-HF with COPD were at significantly greater risk as compared to those without. Treatment with Dapagliflozin showed reduction in the risk of CV death and worsening HF compared to placebo, correspondingly, in patients with and without COPD.
|
|
No COPD |
COPD |
|
||
|
|
Placebo (N=2,085) |
Dapagliflozin (N=2,074) |
Placebo (N=286) |
Dapagliflozin (N=299) |
interaction p-value |
|
Primary outcome |
|
|
|
|
|
|
Events (%) |
419 (20.1) |
325 (15.7) |
83 (29.0) |
61 (20.4) |
|
|
Event rate/100 pt. yrs. |
14.9 (13.5-16.4) |
11.2 (10.1-12.5) |
22.8 (18.4-28.3) |
15.3 (11.9-19.7) |
|
|
HR |
0.76 (0.65-0.87) |
0.67 (0.48-0.93) |
0.467 |
||
|
All-cause death |
|
|
|
|
|
|
Events (%) |
272 (13.1) |
226 (10.9) |
57 (19.9) |
50 (16.7) |
|
|
Event rate/100 pt. yrs |
9.1 (8.1-10.2) |
7.5 (6.6-8.5) |
13.8 (10.7-17.9) |
11.7 (8.8-15.4) |
|
|
HR |
0.83 (0.69-0.99) |
0.83 (0.57-1.22) |
0.956 |
||
Hazard ratios (HR) stratified by baseline diabetes status. Primary composite outcome adjusted for previous HF hospitalization.
Effects of canagliflozin in patients with type 2 diabetes and chronic heart failure: a randomized trial (CANDLE)
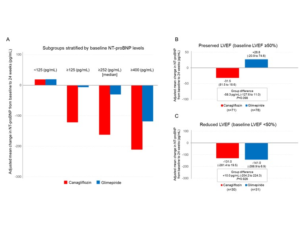
Tanaka A, presented a study at the ESC Congress 2020: A digital experience, which compared the impact of Canagliflozin with Glimepiride, based on changes in N-terminal pro-brain natriuretic peptide (NT-proBNP), in that patient population.
An investigator-initiated, multicenter, prospective, randomized, open-label, blinded-endpoint trial was conducted at 34 centers in Japan. Patients with T2D and clinically stable CHF excluding NYHA class IV, randomized to acquire Canagliflozin 100 mg or Glimepiride (starting dose: 0.5 mg), were analysed by the primary endpoint of non-inferiority of Canagliflozin versus Glimepiride, described as a margin of 1.1 in the upper-limit of the 2-sided 95% confidence interval (95% CI) for the group ratio of percentage change in NT-proBNP at 24 weeks. Data analysis of 233 patients (mean age 68.6 ± 10.1 yrs; 75% male) exhibited mean left ventricular ejection fraction (LVEF) of 57.6 ± 14.6%, with 71% of patients with a preserved LVEF (≥50%). The ratio of NT-proBNP percentage difference was 0.48 (95% CI, -0.13 to 1.59, p=0.226), and hence did not meet the prespecified non-inferiority margin. But, data stratified as per the baseline NT-proBNP levels exhibited a trend that Canagliflozin therapy decreased NT-proBNP levels to a higher level as compared to subgroups with increased levels of NT-proBNP (Figure A). In addition, NT-proBNP levels in the Canagliflozin group exhibited lower a nonsubstantial trend in the subgroup with preserved LVEF (Figure B), however not in the subgroup with decresed LVEF (Figure C). Furthermore, the changes in the NYHA class were comparable among groups (p=0.061) in the overall group, whereas in the subgroup with a preserved LVEF Canagliflozin showed substantial enhancement in NYHA classes as compared to Glimepiride treatment (p=0.027).
Conclusion: This trial did not meet the predefined primary endpoint of changes in NT-proBNP levels, with 24 weeks of therapy with Canagliflozin relative to Glimepiride which together with other recent studies would question the value of continuing to analyse NT-proBNP levels following the initial diagnosis of heart failure. However, there was a non-significant trend for Canagliflozin therapy to decrease NT-proBNP levels in a subgroup with preserved LVEF and enhance symptoms even in stable HF patients.
Myocarditis-associated necrotizing coronary vasculitis: incidence, cause and outcome
Necrotizing coronary vasculitis (NCV) is a rare entity generally correlated to myocarditis (M), which occurrence, cause and responsiveness to treatment is unreported. Thus, Frustaci A, presented an ePoster at the ESC Congress 2020: A digital experience, to report the experience in the diagnosis and management of myocarditis-correlated necrotizing coronary vasculitis (M-NCV) and patients’ outcome.
1916 patients from the 5180 patients gained a histological diagnosis of myocarditis with correlated NCV in 30 (12F, 18M, mean age 47.7 years±15) which were undergoing endomyocardial biopsy in institution. NVC endomyocardial samples were retroactively analysed with, immunohistochemistry for Toll like Receptor 4 (TLR4) and real-time PCR for viral genomes. Serum samples were developed for anti-heart autoantibodies and inflammatory cytokine profile (ELLA assay). M-NCV patients were followed for 6-months with resting ECG, Holter monitoring, 2D-echo and in 45% of cases with cardiac magnetic resonance. Increase of ≥10% in left ventricular EF was categorised as response to treatment. M-NCV patients were compared with a control group of 60 following patients with virus + and – lymphocithic myocarditis and a control group of 30 patients undergoing surgical repair with mitral stenosis and normal LV size and function.
26 M-NCV patients showed heart failure or cardiogenic shock; 3 patients showed electrical instability; 1 patient showed infarct-like symptoms. Cause of M-NCV comprised of infectious agents (PVB19, HHV2, EBV and co-infection of Toxoplasma gondii and PVB19) in 4 patients; chest trauma in 1 patient; drug-hypersensitivity in 2 patients; hypereosinophilic syndrome in 1 patient; primary autoimmune disease in 7 patients; giant cells in 3 patients; although it was arising in the remaining cases. CMR imaging did not diagnose any qualitative difference among M-NVC and M patients. Anti-heart autoantibodies were positive in immune-mediated M-NCV and virus- M; a cross-reaction was found with vessel walls in M-NCV patients in which anti-heart autoantibodies were positive. Myocardial expression of TLR4 was high in the immune-mediated forms and negative in the viral. More increased (p< 0.001) interleukins 1-beta was seen in patients with M-NCV as compared to virus-M patients. M-NCV patients showed a more acute clinical profile with 24% in-hospital death as compared to 1.5% of the M group. Immunosuppression exhibited an enhancement of cardiac function in 86% of M-NCV and 88% of virus-M group.
Conclusion: NCV can be histologically identified in up to 1.5% of a large population with myocarditis. Major causes incorporated viral, autoreactive and autoimmune activities. Intra-hospital death is high (24%). Presence of an immunologic pathway is correlated with an advantageous reaction to immunosuppression.
Temporal characteristics of an integrated diagnostics risk score before and after heart failure hospitalizations in a large real-world population of patients with implantable devices
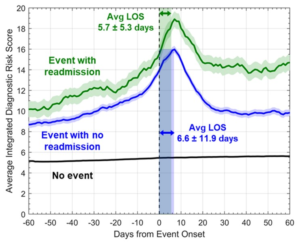
Sarkar S, at the ESC Congress 2020: A digital experience, presented a study, which analysed the temporal characteristics of a integrated diagnostic risk score before and after heart failure (HF) occurrences in a large real-world group of patients with ICD/CRTD devices.
A de-identified database of accumulated electronic health record (EHR) data (2007-2017) was associated with a manufacturer’s device database with continuous diagnostic monitoring data. Patients with ICD/CRTD implants with intra-thoracic impedance diagnostic feature were enrolled in the study. In a Bayesian Belief Network framework, the previously described integrated diagnostic risk score was obtained with integration of daily diagnostic data, including intra-thoracic impedance, night-time heart rate, activity, heart rate variability, and atrial fibrillation (AF) burden, ventricular rate in AF, CRT pacing, ventricular tachycardia episodes and shocks. A total of 17,886 patients with 1.8±1.2 years of follow-up conformed inclusion criteria. The average age of patients was 66.6 ±12.3 years, with 72% being males, and 51% with ICD devices. The average integrated diagnostics risk score with and without readmission in HF occurrences and with no occurrences is exhibited in Figure. A total of 1174 patients showed 1425 HF occurrences with no readmission for HF within 60 days and 282 patients showed 295 HF occurrences which were accompanied with readmission for HF within 60 days. A total of 17,839 patients showed no HF occurrences over 86,858 follow-up months. The average daily risk score was greater over all patients on all 60 days pre and post HF occurrence with readmission as compared to HF occurrences with no readmission (p<0.001) and both were greater as compared to follow-up period with no occurrences (p<0.001). The risk score retrieves less frequently after HF occurrences which are followed with readmission within 60 days as compared to HF occurrences with no readmission.
Conclusion: The average integrated diagnostics risk score was greater before and after HF occurrences with readmission as compared to HF occurrences with no readmission in a large real-world population of patients with ICD/CRTD devices. Re-admissions are more probably in patients with smaller risk score recovery following HF occurrences.
Donation after circulatory death hearts recipients compared to donation after brain death heart recipients have comparable systolic left ventricular function and better myocardial strain at 1 year
Ciarka A, at ESC Congress 2020: A digital experience presented a study which examined systolic performance of the left ventricle with echocardiography with myocardial deformation imaging in donation after circulatory death (DCD) and donation after brain death (DBD) cardiac receivers at 1 year follow up. 46 following DCD cardiac receivers were identified who were transplanted from February 2015 to August 2018 and coordinated them with 46 DBD cardiac receivers. 6 and 7 patients from DCD and DBD group, respectively, died in the first-year post transplant. In the remaining patients, the classical echocardiographic estimations as well as global longitudinal strain (GLS) and global circumferential strain (GCS) were compared at 1-year follow-up.
DCD and DBD patients did not showed differences in terms of classical echocardiographic parameters of left ventricular (LV) structure and systolic function at 1-year follow-up. LVEDV was similar in DCD and DBD patients (101+/-24 vs. 95+/-32 ml, p=0.4 respectively), as well as LVESV (42+/-13 vs. 42 +/- 16 ml, p=0.9, respectively), LV ejection fraction (58+/- 6 vs. 56+/-8 %, p=0.22) and LV mass (156+/-39 vs. 163+/-38 gr, p=0.2, respectively). In contrast, myocardial deformation parameters, such as GLS and GCS, were better in DCD than in DBD (16.1 vs. -14.5%, p<0.01; and -25.2 vs. 22.3%, p<0.05, respectively). E wave velocity, a wave velocity and deceleration time of mitral inflow indicated the diastolic LV function parameters were similar in DCD and DBD group, however E over E prime was lower in DCD as compared to DBD recipients (7.7 +/- 8.7, p<0.05). Fractional area change of the right ventricle was higher in DCD as compared to DBD (46+/-7 vs. 40 +/-7 %, p<0.01) although right atrial volume index was lower in DCD as compared to DBD (25+/- 8 vs. 29+/-9 ml/m2, p<0.01). Other parameters of RV function (systolic excursion of the tricuspid annulus, TAPSE) were similar in both groups.
Conclusion: DCD and DBD heart receivers examined with classical echocardiographic parameters showed similar systolic LV function at 1-year follow. DCD cardiac receivers estimated with the speckle tracking exhibited better myocardial deformation parameters, better systolic right ventricular function and lower filling pressures of the left ventricle.
My patient with diabetes and chronic kidney disease – Should I be looking for macrovascular disease? – What do the Guidelines say.
Fillipptos presented a session on My patient with diabetes and chronic kidney disease – Should I be looking for macrovascular disease? – What do the Guidelines say, at the ESC Congress 2020: A digital experience. In asymptomatic patients with diabetes with or without CKD, the hazard ratios for vascular outcomes are greater. Guidelines routinely recommend the assessment of microalbuminuria to identify patients at risk for renal dysfunction or at a future risk of high CVD. A resting ECG is indicated in patients diagnosed with DM diagnosed with hypertension or with suspected CVD. Assessment of carotid and femoral plaque burden with arterial sonography should be considered as a risk modifier in asymptomatic patients with DM. CAC score with CT may be considered as a risk modifier in the CV risk assessment of asymptomatic patients with DM at moderate risk. CTCA or fucntional imaging radionuclide myocardial perfusion imaging, stress cardiac magnetic resonance imaging or exercise or pharmacological stress echocardiography may be considered in asymptomatic patients with DM for screening of CAD. Screening for asymptomatic CAD in patients with DM remains controversial. In a meta-analysis of any cardiac event like cardiac death, non-fatal myocardial infarction, hospitalisation for heart failure, unstable angina in asymptomatic patients with DM is in favour of screening. Fillipatos was of the opinion that American Diabetes Association (ADA) 2020 guidelines were more discouraging as they do not recommend routine screening of asymptomatic patients for CVD, as it does not improve outcomes as long as atherosclerotic cardiovascular risk factors are treated. ADA considers investigations in the presence of atypical cardiac symptoms or signs and symptoms of associated vascular disease. The risk of CAD increases as the progression of renal disease takes place.
Conclusion: Conclusively, nothing concrete is published, but regular assessment for atherosclerotic CVD risk should be distinguished from sceening for asymptomatic CAD. In absence of evidence that pre-emptive coronary revascularization is effective in reducing death or risk in asymptomatic patients, screening for underlying anatomic CAD lacks either a rationale or evidence-even in at risk asymptomatic patients. In the symptomatic patient or asymptomatic potential transplant recepient, functional stress testing and noninvasive coronary imaging are used to quantify the burden of atherosclerosis, evaulate prognosis, and risk stratify individuals for coronary revascularization or medical optimization.

Effects of liraglutide and semaglutide on stroke subtypes in patients with type 2 diabetes: a post hoc analysis of the LEADER, SUSTAIN 6 and PIONEER 6 trials
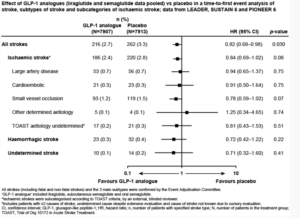
Strain, presented an ePoster at the ESC Congress, 2020: A digital experience, which examined the effect of liraglutide and semaglutide on stroke and its subtypes based on pooled data from LEADER, SUSTAIN 6 and PIONEER 6.
LEADER (NCT01179048), SUSTAIN 6 (NCT0172044 6 ) and PIONEER 6 (NCT02 6 9271 6 ) were global randomised cardiovascular (CV) outcomes trials of liraglutide, subcutaneous semaglutide and oral semaglutide, respectively, in patients with T2D at high CV risk. In this post hoc analysis, the effect of these GLP-1 analogue treatments (pooled) on time to first occurrence of all strokes and subtypes of stroke was evaluated. As per the TOAST classification, ischaemic stroke was subcategorised based on aetiology by an external blinded reviewer. A Cox proportional hazards model stratified by trial with pooled treatment as a factor was used to examine treatment effects. It was observed that across the three trials, lower number of patients 216 /7907 (2.7%) in the GLP-1 analogue group had stroke compared to 262/7913 (3.3%) in the placebo group. GLP-1 analogue significatly reduced the risk of first occurrence of all strokes versus placebo group (HR 0.82, 95% CI 0. 6 8–0.98; p=0.030). Treatment effects were consistent across stroke subtypes: ischaemic (HR 0.84, 95% CI 0. 6 9–1.02; p=0.08), haemorrhagic (HR 0.72, 95% CI 0.42–1.22; p=0.22) and undetermined (HR 0.71, 95% CI 0.32–1. 6 0; p=0.41; Figure). GLP-1 analogue treatment had the greatest advantage versus placebo in small vessel occlusion strokes compared with large artery disease or cardioembolic strokes across the TOAST subcategories; however, no statistically significant effects were found in any subcategory.
Conclusion: In this post hoc analysis of the LEADER, SUSTAIN 6 and PIONEER 6 trials, GLP-1 analogue treatment reduced the risk of stroke in patients with T2D and high CV risk, with an indication using TOAST criteria of the strongest effect on stroke caused by small vessel occlusion.
PARALLAX: Sacubitril/Valsartan versus Individualized RAAS Blockade in Patients with HFpEF
Prof. Burket presented the PARALLAX trial today at ESC Congress 2020: A digital experience. The research team led by Burkert M, et al. randomized a total of 2,572 HFpEF patients to sacubitril/valsartan or their current RAS medication (enalapril, valsartan or placebo if they were not taking a RAS inhibitor). Patients in the trial had a mean age of 73 years and 51% were women. The mean left ventricular ejection fraction at baseline was 56%.
Overall results observed were that patients treated with sacubitril/valsartan showed a significant 16.4% greater reduction in NT-proBNP than those patients treated with optimal individualized medical therapy after 12 weeks (p<0.0001). However, there was no significant difference between groups in terms of improvement in six-minute walk distance at 24 weeks. Both groups saw improvements compared with baseline (mean change was 9.7 m in the sacubitril/valsartan group and 12.2 m in the individualized medical therapy group). In other findings, quality of life improved in both groups and was better in the sacubitril/valsartan group at week four but there was no difference between groups at week 24. Overall, except for heart failure events, serious adverse events were reported in similar proportions of patients in both groups. Heart failure events (such as worsening heart failure requiring hospitalisation or not necessitating hospital admission) were the most common serious adverse events and occurred in more patients in the individualised medical therapy group than in the sacubitril/valsartan group. Based on this, a post hoc analysis showed that sacubitril/valsartan reduced the risk for heart failure hospitalisation by 50% (p=0.005). Patients in the sacubitril/valsartan group also had a significantly lower decline in renal function (estimated glomerular filtration rate; eGFR) at 24 weeks.
Conclusion: PARALLAX study concluded that sacubitril/valsartan reduces NT-proBNP, a biomarker predictive of long-term clinical outcomes in heart failure, but does not improve functional capacity compared to individualized background therapy in patients with heart failure with preserved ejection fraction.