EMPEROR-Pooled: Effect of Empagliflozin on Serious Adverse Renal Outcomes in Chronic Heart Failure – A Prospective Alpha-Protected, Individual Patient-Level Pooled Analysis
Packer M, presented the pooled analysis of EMPEROR-Reduced and EMPEROR-Preserved at the ESC Congress 2021: The Digital experience. Both these randomised trials have showed the advantageous impacts of empagliflozin in patients with heart failure (HF) with a reduced and preserved ejection fraction.
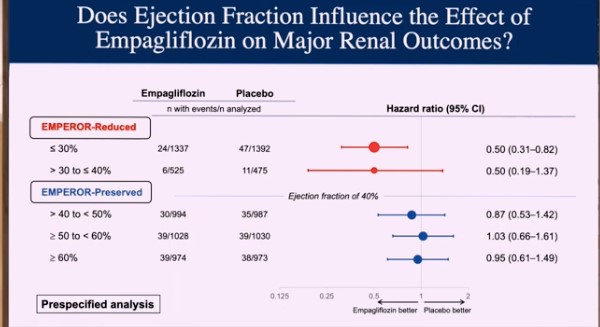
The current study examination pooled the results of these two trials on an individual patient basis. Because of the parallels between the two studies, this was achievable. Both studies compared the effects of empagliflozin against placebo in individuals with documented HF who were receiving all recommended therapies. This study was prospectively designed, and a statistical strategy was devised before enrolling any patients in either trial. The assessment was alpha-protected, which means that the endpoints were statistically powerful and unbiased since the pooled analysis was specified in the individual trials, preventing an exaggerated false positive error rate. The study comprised a total of 9,718 patients. The study found that empagliflozin reduced the risk of HF hospitalisation to a comparable extent (approximately 30% risk reduction) in both EMPEROR-Preserved and EMPEROR-Reduced. The magnitude of the effect on HF hospitalizations was similar throughout a wide range of ejection fractions < 65%, with the drug effect attenuating at higher ejection fractions (65% or greater). At ejection fractions of 40–60%, the effect magnitude appeared to be greater with empagliflozin than in the PARAGON-HF study, which compared sacubitril/valsartan to valsartan. The analysis also revealed that empagliflozin reduced the risk of major renal outcomes in the EMPEROR-Reduced group but not in the EMPEROR-Preserved group. However, when renal outcomes were defined more stringently in EMPEROR-Preserved, pre-treatment ejection fraction impacted the effect of empagliflozin on renal outcomes in a way that matched the drug’s effect on heart failure hospitalisations.
The results showed the advantages of empagliflozin over a broad range of patients with heart failure with a reduced and preserved ejection fraction, incorporating many not effectively managed with currently available agents.
EMPEROR-Preserved: Effect of Empagliflozin on Cardiovascular Death and Heart Failure Hospitalisations in Patients with Heart Failure with a Preserved Ejection Fraction, with and without Diabetes
Anker S, presented the results of “EMPEROR-Preserved: Effect of Empagliflozin on Cardiovascular Death and Heart Failure Hospitalisations in Patients with Heart Failure with a Preserved Ejection Fraction (HFpEF), with and without Diabetes” at the ESC Congress 2021 which was one of the most awaited results.
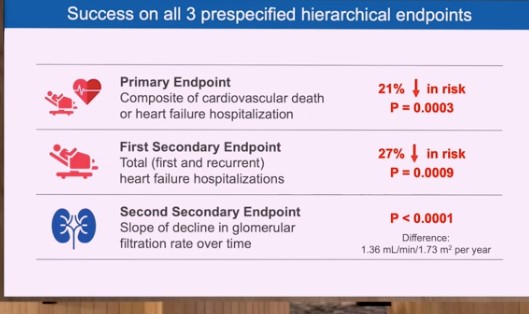
EMPEROR-Preserved analysed the impacts of SGLT2 inhibition in HFpEF patients with and without diabetes. 5,988 symptomatic HFpEF patients (left ventricular ejection fraction > 40%) with and without type 2 diabetes (T2D) were included in the trial from 622 centers in 23 countries. On top of all recommended therapies for HFpEF and co-morbidities, participants were randomly assigned in a 1:1 ratio to receive empagliflozin 10 mg daily or placebo. The primary endpoint was a composite of cardiovascular (CV) death or hospitalisation for heart failure (HF). The first secondary outcome was hospitalisations for HF, including first and recurrent events. The second secondary outcome was the rate of decline in the glomerular filtration rate (eGFR) during study treatment. A primary outcome event occurred in 415 of 2,997 patients (13.8%) in the empagliflozin group and 511 of 2,991 patients (17.1%) in the placebo group (6.9 vs. 8.7 events per 100 patient-years; hazard ratio [HR] 0.79; 95 % confidence interval [CI] 0.69–0.90; p=0.0003) during a median follow-up of 26 months, resulting in a 21% lower risk of CV death or hospital hospitalisation for HF in the empagliflozin group. The impact on the primary outcome were found in all prespecified subgroups, including patients with or without diabetes and those with a left ventricular ejection fraction of less than 50%, 50% to less than 60%, or 60% or more. Regarding secondary outcomes, there was 27% reduction in total (first and recurrent) HF hospitalisations (p=0.0009). When compared to the placebo group, the rate of reduction in eGFR was slower in the empagliflozin group (–1.25 vs. –2.62 ml/min/1.73 m2/year; p<0.0001).
EMPEROR-Preserved trial is the first trial to exhibit unequivocal clinical advantages with a drug in patients with heart failure and a preserved ejection fraction. The trial exhibited that Empagliflozin decreases the risk of a composite of cardiovascular death or hospitalisation for heart failure in patients with heart failure and a preserved ejection fraction with or without diabetes.
Low-dose Rivaroxaban During the Acute Phase of ACS – H-REPLACE Trial
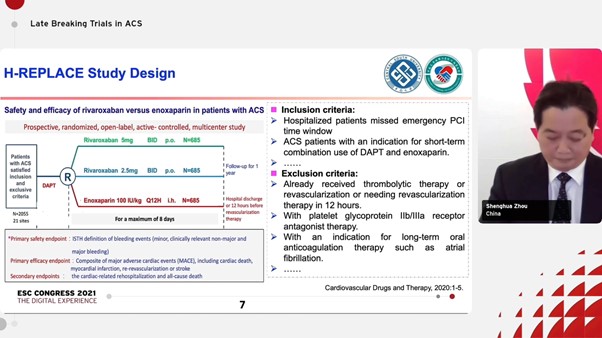
Zhou S et.al presented at the ESC Congress 2021 on safety and efficacy of LMWH versus rivaroxaban in Chinese patients hospitalized with acute coronary syndrome (H- Replace). Nearly half of AMI patients may kiss the primary reperfusion therapy window in the PCI era. In China, only about one third STEMI patients received primary PCI in recent 5 years and most of else patients had to receive delayed revascularization.
As per 2017 ESC guidelines, Enoxaparin is recommended in ACS patients missed emergency revascularization for the duration of hospital stay up to 8 days. The guidelines also state that in low bleeding-risk ACS patients who receive aspirin and clopidogrel, low-dose rivaroxaban (2.5 mg twice daily) may be considered. Mentioned below is the study design for H-REPLACE:
The primary efficacy endpoint was composite of major adverse cardiac events (MACE), including cardiac death, myocardial infarction, re-vascularization or stroke. The primary safety endpoint was ISTH definition of bleeding events. Low-dose rivaroxaban administered during the acute phase of ACS, for patients missed the primary reperfusion window and before selective revascularization, is as safe as enoxaparin but associated with a reduction trend in the risk of MACE compared with enoxaparin during the 6-month follow-up.
Low-dose direct oral anticoagulant rivaroxaban might be a potential alternative of enoxaparin for patients with ACS in the acute phase, who missed the primary reperfusion window and before selective revascularization.
HUYGENS: Evolocumab and Changes in Plaque Composition on OCT
Nicholls SJ et.al. presented at ESC Congress 2021 on the topic of assessing the impact of PCSK9 inhibition on coronary plaque phenotype with optical coherence tomography; highlighting the primary results of HUYGENS study.
It has been found that post ACS, many patients are not being treated with intensive lipid lowering agents despite established clinical benefits of the same. IVUS trials have demonstrated that high intensity statins with and without PCSK9 inhibitors produce plaque regression with the degree of benefit directly proportional to LDL-C lowering. HUYGENS aimed to determine the impact of adding a PCSK9 inhibitor to statin therapy on plaque phenotype after an ACS. The HUYGENS study was designed as follows:
The primary and secondary endpoints were as follows:
- Change in minimum FCT anywhere in the segment
- % change in miniumum FCT
- Change in average minimum FCT
- Change in maximum lipid etc.
Baseline demographics were age, gender, BMI, diabetes, smoking, prior statin use > 4 weeks.
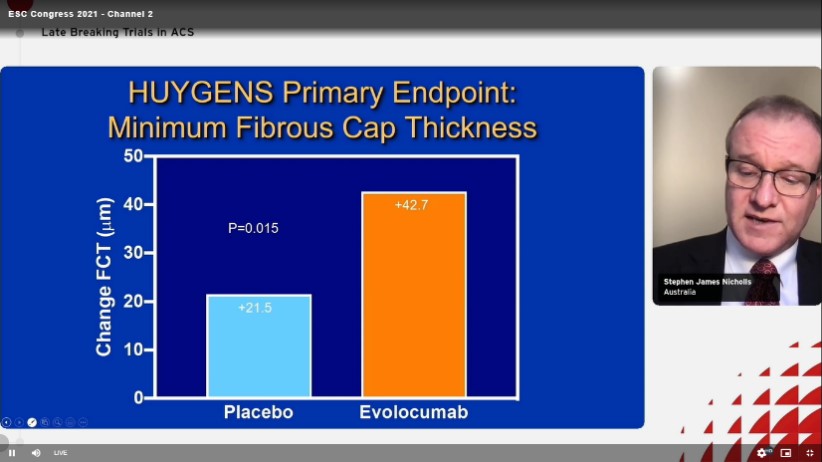
The HUYGENS primary endpoint of minimum fibrous cap thickness were as follows
The study also showed positive results of Evolocumab w.r.t mean minimum fibrous cap thickness. In patients with NSTEMI, more intensive lipid lowering with addition of the PCSK9 inhibitor, evolocumab, for 12 months to maximally tolerated statins resulted in greater increases in the minimum FCT and decreases in the maximum lipid arc, both throughout the vessel and within lipid rich plaques.
At 12 months, after a NSTEMI only 12.5% of patients treated with evolocumab in addition to maximally tolerated statins demonstrated evidence of any region with a minimum FCT < 65 µm, a feature associated with a high risk of plaque rupture. The degree of benefit was directly proportional to the intensity of lipid lowering observed.
With the benefits of lipid lowering on plaque burden have been well established, these findings suggest that this can also favourable modulate plaque phenotype and are likely to contribute to early reductions in cardiovascular risk.
Quantum Genomics Firibastat (QGC001) or Ramipril after Acute Myocardial Infarction for Prevention of Left Ventricular Dysfunction
Montalesot G, presented the results of Quantum trial at ESC Congress 2021. He talked about Brain Aminopeptidase A inhibition with Firibastat to prevent left ventricular dysfunction after acute myocardial infarction.
The primary objective of QUORAM was to compare the effects of two oral doses of Firibastat to those of ramipril on change from baseline in Left Ventricular Ejection Fraction (LVEF) assessed by cardiac MRI in 12 week treatment. It was a multicentre-randomized double blind active controlled phase 2 study with primary endpoint being change in baseline from 12 weeks in LEVF assessed by cardiac MRI (cMRI). The subjects were up titrated if systolic BP ≥ 110mmHg. Other MRI parameters calculated were, end diastolic volume, end systolic volume, and infarct mass.
The Major Adverse Cardiac Events (MACE- Centrally adjudicated) were, cardiovascular deaths, New MI, and cardiac hospitalization. The study also assessed safety w.r.t systolic BP with firibastat 100mg BID, Firibastat 500mg BID, and Ramipril 5 mg BID. Safety w.r.t. change in baseline laboratory tests such as sodium, potassium, eGFR and blood glucose were also assessed.
The study concluded that Firibastat (100mg BID or 500mg BID) was not superior to active comparator ramipril (5 mg BID) to prevent left ventricular dysfunction after first acute anterior MI.
Firibastat depicts global safety profile similar to ramipril and also demonstrates a better hemodynamic safety profile than ramipril.
Age-specific Effects of Blood Pressure-lowering Pharmacotherapy for Prevention of Cardiovascular Disease
The effects of pharmacological lowering of blood pressure (BP) on cardiovascular (CV) influences in older patients were unclear, particularly when BP is not substantially increased. Rahimi K, presented a session at the European Society of Cardiology (ESC) Congress 2021: The Digital Experience on 27th August 2021 which evaluated this issue, by an individual participant-level data meta-analysis of randomised controlled trials (RCTs) of BP-lowering therapy.
Data of 51 RCTs and 358,707 participants was included in the meta-analysis were gathered from the Blood Pressure Lowering Treatment Triallists’ Collaboration and compared pharmacological BP-lowering therapy vs. placebo or other BP-lowering medications or compared more vs. less intensive strategies. Data were collected and patients categorised into baseline age groups and BP ranges (10 mmHg increase from <120 to >=170 mmHg for systolic BP and from <70 to >=110 mmHg for diastolic BP).
The participant’s age was ranged from 21 to 105 years at randomisation (median 65, interquartile range 59 to 75), with 42,960 (12%) participants aged <55 years, 128,437 (35.8%) aged 55 to 64 years, 128,506 (35.8%) aged 65 to 74 years, 54,016 (15.1%) aged 75–84 years, and 4,788 (1.3%) aged >=85 years.
Age groups showed efficient pharmacological BP reduction, with no evidence to recommend that relative risk reductions for prevention of major CV events differed with baseline systolic or diastolic BP levels, below to <120/70 mmHg. While there was a suggestive proof for decreasing relative risk reductions with increasing age (adjusted p for heterogeneity = 0.05) and limited statistical power in the oldest age group (90 years at the end of the study) in segregation, however, absolute risk reductions did not follow the same pattern and emerged to be even larger in the older age groups. Stratified impacts on all-cause death followed a similar pattern, with no evidence to recommend that therapy elevated mortality in any age group. This most comprehensive study of the age- and BP-stratified effect of antihypertensive medication yields enthralling evidence for the efficacy of pharmacological BP decrease into old age irrespective of baseline systolic or diastolic BP.
Antihypertensive medication should be considered as a substantial treatment option, regardless of age, with removal of age-associated thresholds from international guidelines.
Late Breaking Science in Heart Failure: DAPA-HF
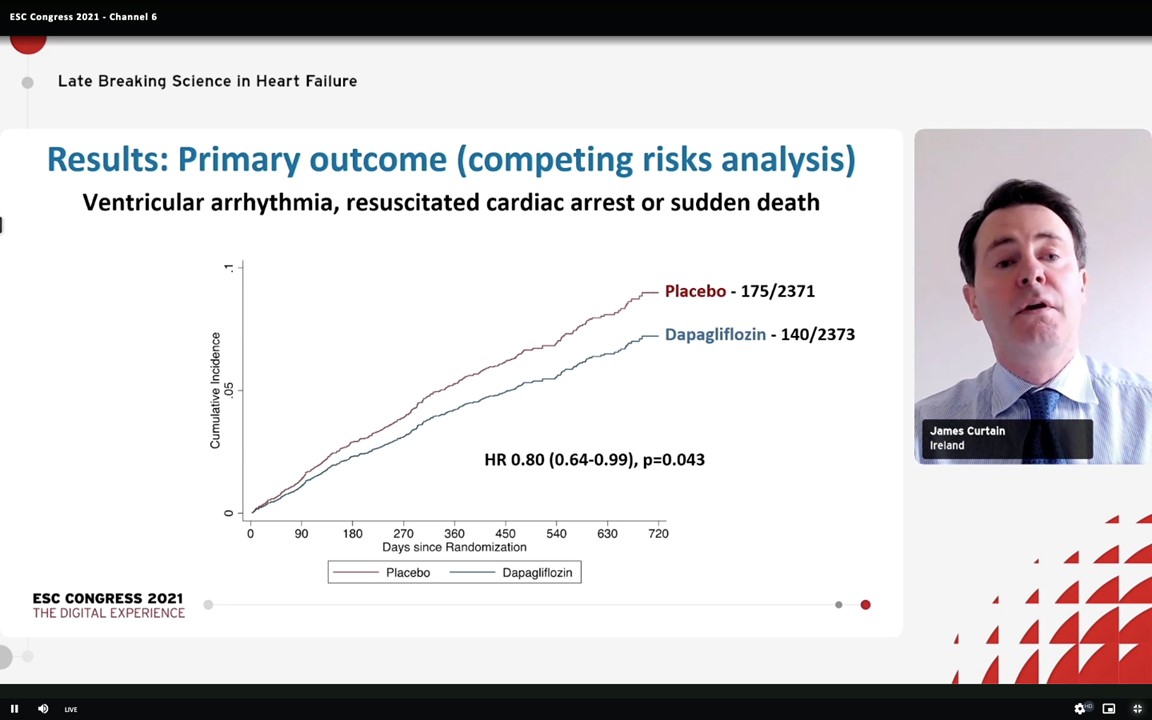
Ventricular arrhythmias are a substantial cause of sudden death in patients with HF with reduced ejection fraction (HFrEF). Curtain J, presented a session at the European Society of Cardiology (ESC) Congress 2021: The Digital Experience on 27th August 2021 which presented findings of a post-hoc examination of the DAPA-HF trial, examining the outcomes of Dapagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor, on a composite of serious ventricular arrhythmia, resuscitated cardiac arrest or sudden death in the patients with HFrEF.
4,744 patients with HFrEF were enlisted in the trial. From the participants, 115 (2.4%) exhibited a serious ventricular arrhythmia, 206 of the 500 (41%) cardiovascular deaths were adjudicated as sudden deaths, and 8 of the 23 (35%) patients who suffered a cardiac arrest were survived. Dapagliflozin therapy showed a significant decrease in the occurrence of the composite outcome (5.9%) as compared to placebo (7.4%) (hazard ratio 0.79; CI 0.63 to 0.99; p=0.037) and the impact of Dapagliflozin on the individual components of the composite outcome was constant. Moreover, Dapagliflozin effects were compatible in patients with an ischaemic and non-ischaemic aetiology.
This investigation constitutes on existing evidence for the favourable impacts of SGLT2 inhibitors in HFrEF and generates one prospective procedure for how these drugs reduce mortality in HFrEF.
Late Breaking Science in Heart Failure: VANISH Trial Results
Sarcomeric hypertrophic cardiomyopathy is related with increased risk of HF, atrial fibrillation and sudden death. Ho C, presented a session at the European Society of Cardiology (ESC) Congress 2021: The Digital Experience on 27th August 2021 which described the findings of the VANISH trial, which examined whether the angiotensin II receptor blocker, valsartan, could slow the development of early-stage sarcomeric hypertrophic cardiomyopathy.
In this multicentre, double-blind trial, 178 patients were randomised to acquire valsartan (n=88) or placebo (n=90). The primary endpoint was a composite Z-score of various estimates of cardiac structure/function, namely: changes in left ventricular (LV) wall thickness, mass and volume; left atrial volumes; tissue Doppler diastolic and systolic velocities; and serum levels of high-sensitivity troponin T and N-terminal pro-B-type natriuretic peptide (NTproBNP).
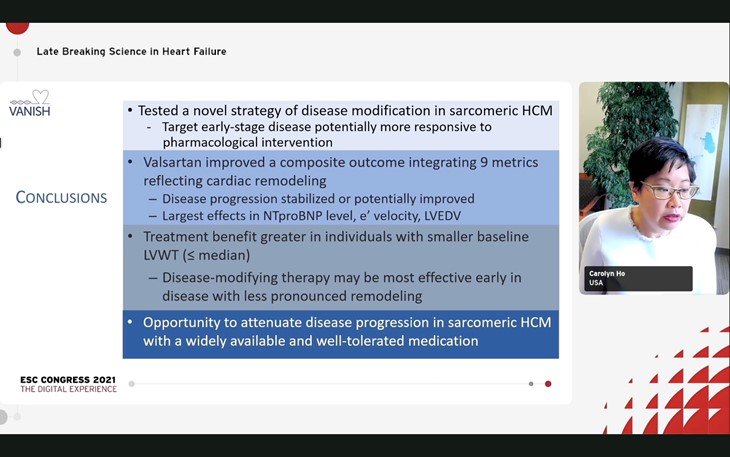
Valsartan showed improvement in the primary endpoint with an increase of 0.231 in the Z-score (confidence interval [CI] 0.098 to 0.34; p=0.001) over a 2-year period. The highest contributors to the development were improved LV end diastolic volume, tissue Doppler diastolic velocity and NTproBNP levels.
Valsartan treatment exhibited a promising positive effects on cardiac structure and function although examining a unique strategy of disorder modification of genetic cardiomyopathy.
Acute Safety and Performance Outcomes from the inspIRE Trial Using a Novel Pulsed Field Ablation System for The Treatment of Paroxysmal Atrial Fibrillation
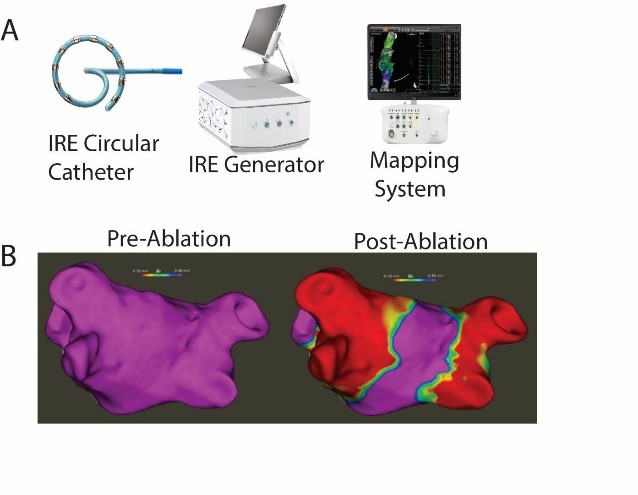
The inspIRE clinical trial was outlined to evaluate the safety and effectiveness of a fully integrated biphasic pulsed field ablation (PFA) system comprised of a multi-channel generator, changeable decapolar irrigated loop circular catheter, and mapping system (Figure A) for the treatment of paroxysmal atrial fibrillation (PAF).
Potter TD, presented a session at the European Society of Cardiology (ESC) Congress 2021: The Digital Experience on 27th August 2021 which conferred the initial viability of electrical pulmonary vein isolation (PVI), procedural execution, and acute safety findings with this novel PFA system in a multicentre clinical trial.
Up to 550 patients were enlisted in inspIRE, a prospective, non-randomized, multi-centre study. PVI is executed with the novel, variable loop circular catheter, compatible mapping system and generator. Acute procedural efficacy (entrance block in all clinically targeted PVs post adenosine/isoproterenol challenge) and the incidence of primary adverse events (PAEs) were evaluated. PAEs are described as the incident of cardiac tamponade/perforation, myocardial infarction, stroke/cerebrovascular accident, thromboembolism, transient ischemic attack, permanent phrenic nerve paralysis, pulmonary edema, pericarditis, and any major vascular access complications within 7 days of the ablation procedure. Furthermore, any occurrence of procedure or device associated death, atrio-esophageal fistula, or PV stenosis (correlated to the ablation procedure or study catheter) within the 12M follow-up period is categorised as a PAE.
A total of 35 PAF patients (age 59.7±10.7 years, 54.3% male) were managed over 5 European sites by 6 operators. Acute procedural success was achieved in 100% of study patients (Figure B) with zero occurence of PAEs. Mean total procedure time was 82.9±19.9 minutes with 27.0±11.9 minutes of PFA from first to last application. Average fluoroscopy use was 10.6±6.8 minutes and LA dwell time was 45.6±15.3 minutes.
Initial findings of the inspIRE trial showed the acute safety and efficacy of the new integrated IRE circular catheter, mapping system and generator for PVI in PAF patients.
Intracoronary Pharmacological Therapy versus Aspiration Thrombectomy in Patients Presenting with ST-segment Elevation Myocardial Infarction (IPAT-STEMI): A Systematic Review and Meta-analysis
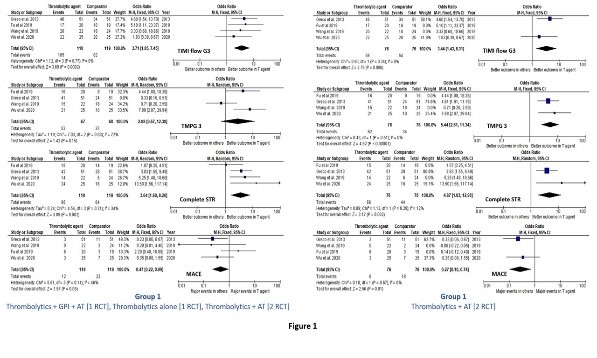
Various strategies have been recommended in patients with MI such as aspiration thrombectomy (AT), and/or localized or intracoronary (IC) therapy with thrombolytic agents and/or glycoprotein IIb/IIIa inhibitors (GPI) for large thrombus load. Kaddoura R, presented a session at the European Society of Cardiology (ESC) Congress 2021: The Digital Experience on 27th August 2021 which analysed the efficacy of IC-administered pharmacological agents alone or combined with AT compared with AT alone as a supplement to percutaneous coronary intervention (PCI) in STEMI patients with large thrombus burden.
The method was performed as per the Cochrane Handbook for Systematic Reviews and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement. A systematic search strategy was executed on February 22, 2020 and updated on February 13, 2021 with MEDLINE, EMBASE, CENTRALE, Scopus, ProQuest Public Health, Web of Science databases. In patients with STEMI who underwent PCI, eligible trials were randomized controlled trials (RCT) comparing IC-administered thrombolytic agents and/or GPI with or without AT to AT alone. The primary outcomes were coronary reperfusion indices e.g., thrombolysis in myocardial infarction (TIMI) flow grade 3 (G3), TIMI myocardial perfusion grade (TMPG) 3, Myocardial blush grade (MBG) 2/3, ST-segment resolution (STR). Others incorported clinical outcomes e.g., major adverse cardiovascular events (MACE).
12 RCT enlisted 1,466 patients were incorporated in the final examination. The trials were classified into 3 groups: (1) Thrombolytics [4 RCT], (2) GPI [3 RCT], and (3) GPI+AT [7 RCT]. Groups 2 and 3 incorporated two mutual studies with multiple arms. Thrombolytic treatment, as presented in 4 RCT, substantially enhanced TIMI flow G3 (OR = 3.71, 95% CI: 1.85-7.45; p overall effect = 0.0002; heterogeneity = 0%), complete STR (OR = 3.64, 95% CI: 1.60-8.26; p overall effect = 0.002; heterogeneity = 34%) and MACE (OR = 0.47, 95% CI: 0.22-0.99; p overall effect = 0.05; heterogeneity = 46%), however not TMPG 3, as compared to AT alone. Combining the data of the two studies with similar intervention i.e., thrombolytics + AT additionally clarified the findings with statistical enhancement of TMPG 3 as well (OR = 5.44, 95% CI: 2.61- 11.34; p overall effect < 0.000001; heterogeneity = 0%) (Fig. 1). Combined findings for GPI and GPI+AT groups did not exhibited statistical enhancement in TIMI flow G3, MBG 2/3, nor complete STR (Fig. 2).
The results of this meta-analysis exhibited that IC-administered thrombolytic agents enhanced myocardial reperfusion and MACE than AT alone in STEMI patients undergoing PCI.
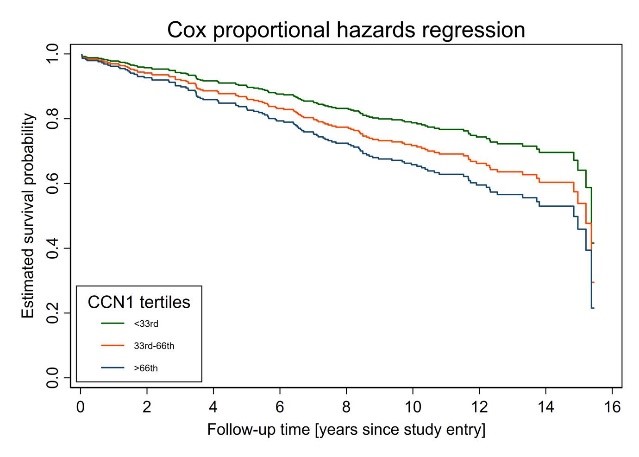
Cysteine-rich Angiogenic Inducer 61 (CCN1) Independently Predicts All-cause Mortality in Patients with Dilated Cardiomyopathy
Cysteine-rich angiogenic inducer 61 (Cyr61, CCN1) is a member of the CCN family of matricellular proteins exerting crucial activities in inflammation and fibrotic turnover. Currently it has been exhibited that CCN1 enhances risk stratification for all-cause mortality in ACS patients. Klingenberg R, presented a session at the European Society of Cardiology (ESC) Congress 2021: The Digital Experience on 27th August 2021 which demonstrated the prognostic value of CCN1 serum levels for survival in a large cohort of DCM patients since both inflammation and fibrosis are vital methods involved in the pathogenesis of dilated cardiomyopathy (DCM).
The group compromised patients with a primary diagnosis of DCM, described as a decreased left ventricular ejection fraction (LVEF < 45%) and an increased left ventricular end diastolic diameter as per the HENRY score (LVEDD according to HENRY > 117%) at the time of diagnosis. Exclusion criteria were primary valvular disorders (≥ second degree), acute myocarditis, active infectious diseases, pulmonary diseases, cancer, chronic alcoholism and heart failure of other origins. An enzyme-linked immunosorbent assay was used to ascertain CCN1 levels in human serum. Multivariable cox regression models for the correlation among CCN1 and all-cause mortality were adjusted for age, sex, disease duration, LVEF, estimated glomerular filtration rate (eGFR) estimated based on the CKD-EPI formula, high-sensitivity C-reactive protein (hs-CRP) and aminoterminal-proB-type natriuretic peptide (NT-proBNP) levels.
306 DCM patients showed available biomarker and clinico-demographic data in this single-center group (79.3 % males) with a mean age of 55.2 years [interquartile range [IQR] 47.9, 64.8]). On average, disorder duration was 0.3 years (IQR 0.9, 1.9), LVEF 31% (IQR 25, 37), LVEDD 67.7 mm (IQR 63, 72), and eGFR 91.5 ml/min (IQR 74.1, 102.4). In a median follow-up of 12.5 years (IQR 10.5, 14.1), a total of 114 (37.3 %) patients were died. Multivariable-adjusted cox regression models showed an elevating all-cause mortality risk over CCN1 tertiles (p for trend = 0.03), with the greatest event in the highest tertile (hazard ratio [HR] 1.75; 95%-CI 1.04, 2.94; P=0.034) than the lowest tertile (Figure 1).
CCN1 forecasts long-term survival in DCM patients individualistic of NT-pro-BNP and other risk factors. Additional research requires to analyse whether this novel biomarker also plays an innovative role in the pathogenesis of DCM.
Clinical Course and Related Costs of Patients with Diabetes and Heart Failure and/or Chronic Kidney Disease, Drawn from a Sample of More Than 7 Million People
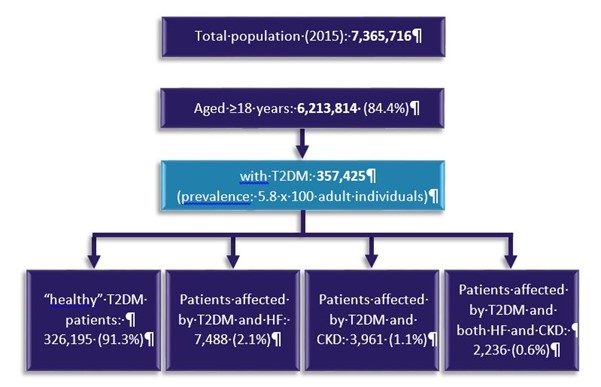
Diabetes (T2DM), heart failure (HF) and chronic kidney disease (CKD) are one of the leading causes of mortality and hospitalization worldwide. Maggioni AP, presented a session at the European Society of Cardiology (ESC) Congress 2021: The Digital Experience on 27th August 2021 which analysed the Ricerca e Salute (ReS) database which is purposed to explain clinical epidemiology, 2-year outcomes and direct costs of T2DM patients with HF, CKD or both in an association setting.
7,365,716 patients were included the ReS database and were assessed. Patients with T2DM were selected and consecutively divide in the following mutually exclusive groups in 2015 (Figure):
- “Healthy” T2DM patients, patients with T2DM however without coronary artery disease (CAD), HF, stroke, TIAs, peripheral artery disease (PAD) and CKD
- Patients affected by T2DM and HF
- Patients affected by T2DM and CKD
- Patients affected by T2DM and both HF and CKD
Table exhibits the baseline characteristics, hospitalization causes, and associated costs of the 4 groups. In the 2-year follow-up, T2DM patients with comorbidities are older, more commonly males, and more frequently admitted for CV and renal reasons. T2DM patients with both HF and CKD showed the worst effect profile. T2DM patients with both HF and CKD exhibited 5 times more the cost per patient per year as compared to patients with T2DM without these comorbidities.
Coexistence of HF and/or CKD in patients with T2DM is correlated with a very high clinical and economical load. The most suitable approach should be to embrace a synergetic way that adopts CV, renal and metabolic diseases in spite of treating each condition independently.
Additive Prognostic Value of Cardiac Biomarkers in Patients with Chronic Obstructive Pulmonary Disease and Heart Failure
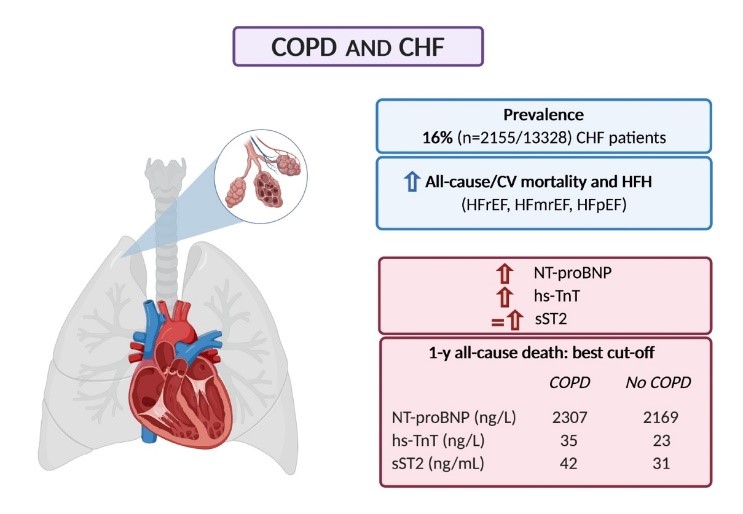
Chronic obstructive pulmonary disease (COPD) is a common comorbidity in patients with heart failure (HF). Vergaro G, presented a session at the European Society of Cardiology (ESC) Congress 2021: The Digital Experience on 27th August 2021 which evaluated the effect of COPD on circulating levels and prognostic value of 3 HF biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity troponin T (hs-TnT), and soluble suppression of tumorigenesis-2 (sST2).
13328 patients with chronic HF, known COPD status and NT-proBNP, hs-TnT, sST2 values were enrolled in the study and their individual data were assessed.
Patients with COPD (n=2155, 16%) were older (age 71 years [64-77] vs. 66 [57-75]; p<0.001), more commonly men (79% vs. 74%; p<0.001), showed more severe dyspnoea (43% in New York Heart Association [NYHA] class III-IV vs. 31%; p<0.001), slightly worse renal function (median estimated glomerular filtration rate [eGFR] 58 mL/min/1.73 m2 [43-73] vs. 60 [46-77]; p<0.001), higher NT-proBNP (1508 ng/L [650-3363] vs. 1239 ng/L [479-2911]; p<0.001), hs-TnT (22 ng/L [13-38] vs. 17 ng/L [9-30]; p<0.001), and sST2 (31 ng/mL [23-45] vs. 29 [21-43]; p=0.040) as compared to patients without COPD. In both the COPD and no-COPD subgroups, the best cut-offs of the 3 biomarkers clarified the prognosis of 1- and 5-year all-cause and cardiovascular mortality and 1- to 12-month HF hospitalization across a prognostic model incorporating age, sex, ischemic aetiology, eGFR, HF categories, NYHA III-IV, beta-blocker use and the NT-proBNP cut-off alone.
Patients with COPD showed greater circulating cardiac biomarkers in patients with HF. Patient categorisation based on COPD-specific cut-offs clarifies risk reclassification for all-cause and cardiovascular mortality and HF hospitalization and might be beneficial for decision making and treatment.

