Autoimmunity and Primary Immunodeficiency
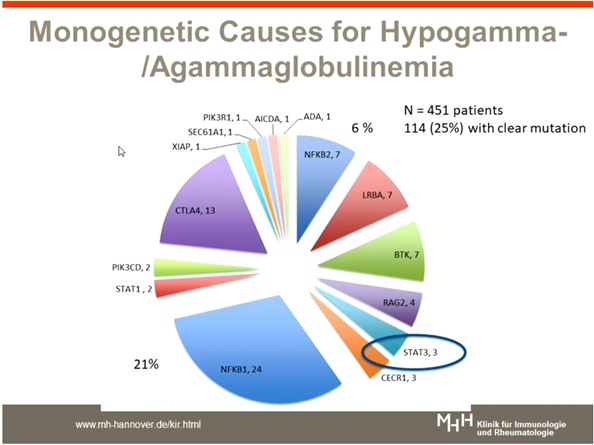
Schmidt RE, presented a study in a session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. Most common are antibody deficiencies, e.g. common variable immunodeficiency (CVID) or X-linked agammaglobulinaemia (XLA). Autoimmunity (AID) in PIDs includes autoimmune cytopenias (e.g. AIHA, ITP), organ specific autoimmunity (thyroiditis or other glandular inflammatory disease, arthritis, myositis), chronic inflammatory bowel diseases (IBD), granulomatous disease (CGD), autoimmune lymphoproliferative syndrome, vasculitis and granulomatous lymphocytic infiltrative lung disease (GLILD). CVID has been defined as primary antibody deficiency (at least low concentrations of IgG and IgA, mostly also of IgM).
Clinical symptoms of CVID are hypogammaglobulinaemia and recurrent, respiratory tract infections, granulomatous lymphocytic interstitial lung disease, autoimmune enteropathy, autoimmune cytopenias and lymphocytic organ infiltration. CTLA-4 is essential inhibitory receptors on Tregs. CTLA-4 captures its ligands CD80 and CD86 from the surface of ACPCs leading to less immunosuppression. LRBA regulates CTLA-4 trafficking in regulatory T cells. Absence of LRBA leads to lysosomal degradation of CTLA-4.
APDS (activated PI3Kδ Syndrome): PI3Kδ gain-of-function results in lymphoproliferation, T cell senescence and immunodeficiency. Leniolisib normalized β cells decreases transitional β cells & increases naïve β cells. Leniolisib improved lymphoproliferation by reducing the volume of spleen & lymph nodes.
In the future with increasing use of functional genetics many ill-defined syndromes of immunodeficiency or chronic inflammatory diseases will be dissolved into genetic categories. Leniolisib is a potent and selective oral PI3Kδ inhibitor and also led to significant improvement in the immunological status and clinical presentation of patients with APDS/PASLI.
Secondary Immunodeficiency in Patients with Autoimmune Disease
Li PH, presented a study in a session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. A 69 year old female present with unremarkable past medical history. She presented with bilateral sensorineural hearing loss, fleeting lung shadows and as elevated anti-MPO. She was diagnosed with granulomatosis with polyangiitis (GPA). High-dose prednisone and rituximab was induced. She was admitted 2 months later for severe community acquired pneumonia. She was managed with i.v meropenem, ganciclovir, and co-trimoxazole, withhold MMF. IVIg loading then 0.4g/kg monthly switched to SCIg weekly home therapy and remains well and infection free since.
Most common secondary immunodeficiencies are predominantly antibody or cellular deficiencies. Secondary antibody deficiency is 30 times more common than primary antibody deficiencies. The most common primary causes of antibody deficiency are single gene and other defects such as ataxia telangiectasia, hyper-IgM syndromes, etc. and chromosomal anomalies. The most common secondary causes of antibody deficiency are drug-induced (Anti-CD20 mAbs (rituximab)), infectious disease, malignancy and other systemic disorders.
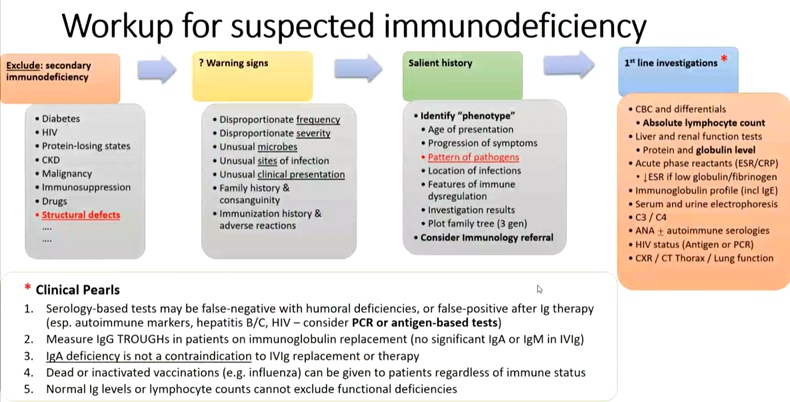
Immunodeficiency can be suspect when observed severe infections, persistent infections despite therapy, unusual sites of infection or microbes, recurrent infections, family history, unusual clinical presentation, unusual phenotype suggestive of syndrome associated with immunodeficiency.. Overarching principles includes (1) patients and their parents/carers should be specifically informed about the possibility and implications of developing hypogammaglobulinaemia secondary to BCTTs. There should be locally agreed pathway for patients to report infections. (2) health-care professionals using BCTTs should be aware of local referral pathways for hypogammaglobulinaemia and its complications. (3) The commencement of IGRT and its route of administration should follow a shared decision-making process between the patients, the clinician supervising the care of the underlying autoimmune disease and a clinical immunology service.
Secondary immunodeficiencies are overlooked and undertreated and may be reversible but many are not. Assessment and decision on treatment requires clinical context and functional Ab testing.
Fatigue in Arthritis, SpA & Systemic Rheumatic Diseases

Primdahl RNJ, presented a study in a session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. Fatigue may cause by pain intensity, disability- to some degree, poor sleep, depression and worries and anxiety. Fatigue may cause physical, cognitive, emotional and behavioural impact such as having heavy flu, even loss of appetite, concentration and memory problems, difficult to plan and be spontaneous, decreases motivation, frustration, feeling sensitive, hopeless, and lack of energy for other activities and social role. Physical fatigue is more prominent than mental fatigue in Lupus.
RA patients may experience fatigue as alone with their fatigue, lack of understanding and professional support is rare, uses language as a tool for improved understanding, necessary prioritisation in everyday life, and time gets another meaning- increased need for rest and sleep. The patient’s own management strategies such as take breaks and relax during the day, plan and prioritise, ask for help, divide tasks over several days to match energy level and taking a day off to recover.
Following option can be used to measure fatigue such as visual analogue scales (VAS) or numerical rating scales (NRS) endpoints can differ, SF-36 vitality subscale, profile of figure (ProF), multidimensional fatigue scale (MFI), functional assessment chronic illness therapy (Fatigue) (FACIT-F), fatigue severity scale (FSS), checklist individual strengths (CIS20R and CIS8R) and chalder fatigue questionnaire (CFQ). Multi-dimensional scales include Bristol Rheumatoid Arthritis Fatigue, Multidimensional Questionnaire (BRAF-MDQ) and Bristol Rheumatoid Arthritis Numerical Rating Scales (BRAF-NRS) for severity, effects and coping.
Treatment with biologics can lead to a small to moderate improvement in fatigue. Non pharmacological management includes support to the patients to increase physical activity, patient education- learn how to self-manage fatigue, pain and sleep, goal setting, problem solving, action planning and communication training, cognitive behavioural therapy and motivational interviewing, activity diaries and guided reflections, and pacing and prioritising, management of fatigue at work.
As clinicians, ask the patients whether they experience fatigue and how it affects their everyday lives, inform about the prevalence, suggest some changes in pharmacological treatment, and offer psycho-social support.
Eight Pillars of Oncorheumatology
Szekanecz E, presented a study in a session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. The goal of the study is to study the correlation between cancer & musculoskeletal diseases. Secondary malignancies in autoimmune inflammatory rheumatic diseases includes (1) long standing autoimmune inflammation: chronic β-cell stimulation-lymphoma (SLE, RA, SSc, SS) and target organ (inflammation, smoking)- solid tumor (RA, SSc, DM). (2) viral virus particles: EBV, terivirus (HTLV)-lymphoma (SLE). Soluble tumours antigen is associated with rheumatic disease, due to they have similar chemical structure. Adhesion molecules can be expressed not only on tumor cells but also on inflammatory cells. Tumor-associated antigens in systemic sclerosis and scleroderma findings includes elevated level of tumor marker was associated equally in patients with SLE & scleroderma.
Possible increase in risk of malignancies after antirheumatic therapy (DMARD, biologics) was reported. Risk factors includes cumulative dose treatment duration, age, smoking. Significantly higher risk of cancer was reported in case patient administrating AZA, cyclosporine or CYC and NASIAD.
Principles of physical therapy includes: multidisciplinary approach, physio-/physical therapy is part of complex musculoskeletal rehabilitation, patient with cancer/after cancer, under on-going oncotherapy/ survivors, individualized risk/benefit assessment like tumor type, stage, remission state and risk factors, maximal safety needed, avoid physical overload, decreased symptoms, increased quality of life & increased survival rates. Lots of mediators are created by the tumor cells which means they are biologically active substances responsible for Immunological manifestations. Paraneoplastic syndromes include vasculitis syndromes, CTDs, arthritis, skin and muscle disorders and metabolic dysfunctions. Immune-checkpoint inhibitors counteract the augmentation of coinhibition, resulting in an efficient antitumor response but also the possibility of autoimmune adverse effects.
The connection between cancer and physiotherapy is also debatable. Musculoskeletal para -neoplastic syndromes can be caused by cancers and their mediators. Immune-related side effects can occur as a result of chemotherapy and immunotherapy (immune checkpoint inhibitors). Rheumatologists must also consider primary and secondary cancers of the musculoskeletal system.
Recent Advances in MRI in Osteoarthritis
Guermazi A, presented a study in a session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. MRI is mostly useful in osteoarthritis because there are several issues with radiography such as positioning issues/repeatability in longitudinal studies and clinical trials, JSN is composite of cartilage and meniscal damage and extrusion (non-specific), not sensitive to change over time, limitations due to projectional nature of the technique and only bone features of OA visualized, OA is multiple disease: inflammation, bone marrow lesions, meniscal damage, etc.
Knee with multiple abnormalities on MRI indicates early stage osteoarthritis, despite lack of radiographic osteoarthritis. Author reported that most study 21% of KL2 knees had no cartilage damage in the MTFJ and 41% in the LTFJ. It was also demonstrated that 25% of KL 2 have severe wide-spread full thickness damage medially. So it concluded that KL 2 and 3 is not a homogenous sample of early-to moderate OA.
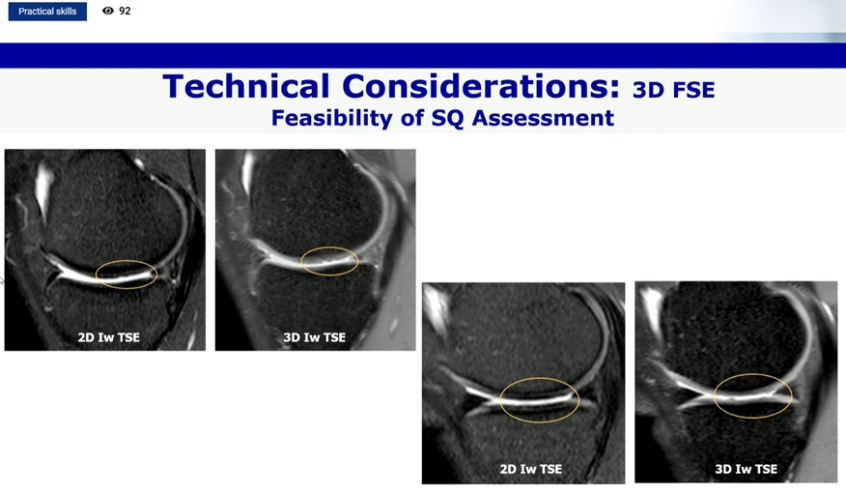
Different imaging approaches to OA joint assessment using MRI are available. Some of them are established like quantitative analysis, semi quantitative analysis, DCE MRI, compositional analysis, bone shape and metabolic imaging. Focal defects were not shown by 3D SPGR-like sequences used for Q-MRI but on morphologic SQ-MRI. Compositional MRI detects cartilage alterations prior surface damage is evident. Changes in GAG, collagen and water content/organization are detectable by sophisticated MRI technique. It was also reported that 3D FSE is faster than 3 consequences 2D FSE and both has similar diagnostic accuracy.
X-ray has low sensitivity and specificity. Different MRI assessment instruments are available such as Q, SQ, compositional, bone shape. Different structural phenotype exit and may progress differently. MRI is being used as an outcome measure in Phase III clinical trials.
Composite of Relevant Endpoints in Sjögren’s Syndrome (CRESS): A Comprehensive Tool for Clinical Trials
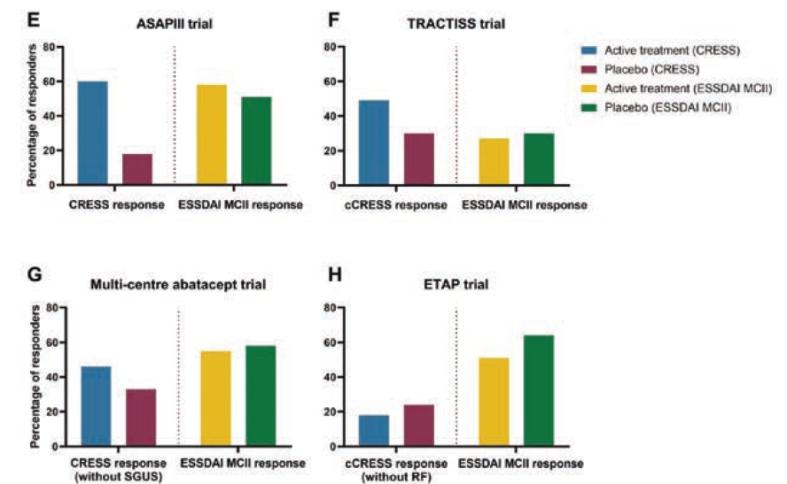
De Wolff L, presented a study in a session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. The study’s aim is to establish and validate the Sjögren’s Syndrome Composite of Relevant Endpoints (CRESS). TRACTISS (rituximab) study, multi-centre abatacept study, and ETAP (tocilizumab) trial were all used to validate CRESS. All four studies had their CRESS response rates measured at the primary endpoint visit.
Systemic disease activity, patient-reported symptoms, tear gland, salivary gland, & serological item were chosen as the five complimentary components that make up CRESS. A whole CRESS answer was defined as a response on at least three of the five elements. At week 24, the CRESS response rates for abatacept were 24/40 (60%) vs. 7/39 (18%) for placebo in the ASAP-III study (p<0.001).
cCRESS response rates for TRACTISS in external validation studies were 33/67 (49%) rituximab vs. 20/66 (30%) placebo at week 48 (p=0.026). At week 24, the multi-centre abatacept trial’s CRESS response rates (without SGUS) were 41/92 (45%) abatacept vs. 30/95 (32%) placebo (p=0.067). At week 24, the cCRESS response rates (without rheumatoid factor) for ETAP were 10/55 (18%) tocilizumab vs. 13/55 (24%) placebo (p=0.482). In RCTs with high baseline ESSDAI scores (>5), CRESS was able to almost halve placebo response rates compared to ESSDAI MCII of 3 points.
In RCTs that previously had unfavourable primary endpoint outcomes, the CRESS observed greater response rates in abatacept and rituximab treated patients compared to placebo. CRESS indicated that there were no differences between tocilizumab and placebo for practically all outcome measures, despite poor response rates. The CRESS is a well balanced, practicable composite endpoint for pSS patients in clinical studies.
Fatigue in Arthritis, SpA & Systemic Rheumatic Diseases (Win) / Treatment of Severe Lupus Nephritis (HOT)
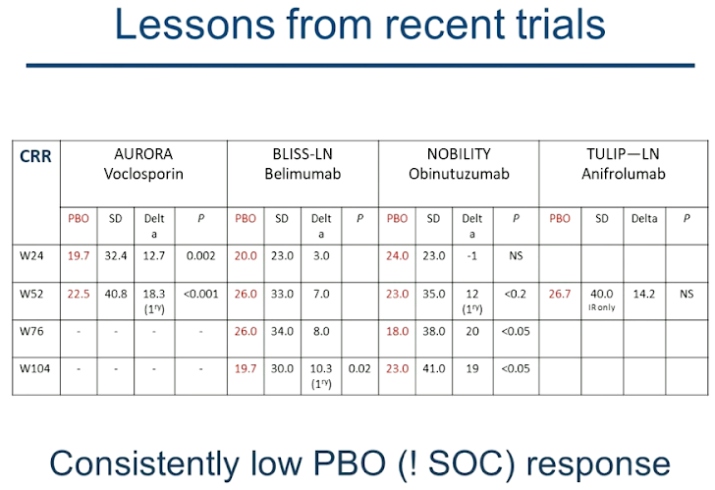
Professor Frederic A. Houssiau presented a study “Treatment of severe Lupus nephritis” in a session at the European Alliance of Associations for Rheumatology, (EULAR) 2021, Scientific program. Treatment of Lupus nephritis (LN) is a race against nephron loss. Current sequential therapy for LN allows for 20-30% complete clinical renal response at 6-12M, 20-25% relapses at 3-5 years, 5-20% ESKD at 10 years and CKD.
Prognosis of LN can be improved by implementing Treat-to-target approach and combination therapy. Treat-to-target approach considers clinical, pathological, and immunopathological targets. Early proteinuria decrease predicts good long term renal outcome. 94% of the patients achieving a proteinuria <0.7g /day at 12 months had a sCr<1mg/dL at 7 years. Also, 69% of patients who might not achieve the target are predicted to have a good long term renal outcome.
The challenge lies in identifying patients who haven’t reached clinical target and will experience a poor longer outcome for whom a change in therapy might be appropriate. AI and CI at baseline don’t predict relapses or renal impairment but AI and CI can predict relapses and impairment respectively at repeat biopsies. Use of calcineurin inhibitors like Voclosporin has its pros as well as cons, thus biologics such as Belimumab, Anifrolumab, and Obinutuzumab have been considered for treatment. However, recent trials indicate a consistently low PBO response. Assessment of immune cell infiltrates or ASC in TI, global and single cell transcriptomes and liquid biopsies might aid in choosing the best treatment course. The treatment must be prescribed based on patients’ extra-renal diseases, specific toxicity profile, drug availability as well as reimbursement criteria.
Treat-to-target approach might be implemented to avoid nephron loss. Per-protocol repeat renal biopsy may be a part of this strategy. A switch from sequential therapy to combination therapy is warranted since current standard of care does not meet the expectations of patients or physicians.
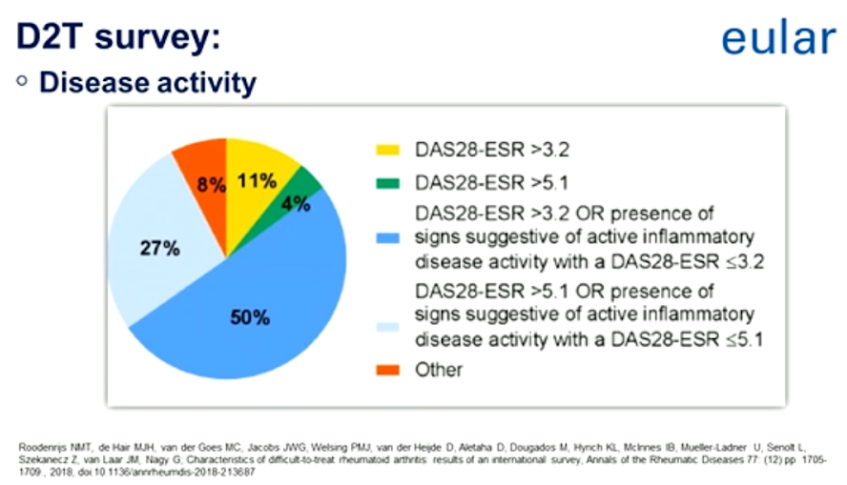
Definition and Recommendation for the Management of Difficult to Treat Rheumatoid Arthritis
Professor, Dr. Gyorgy Nagy, presented a study “EULAR definition of difficult to treat Rheumatoid Arthritis” in a session at the European Alliance of Associations for Rheumatology, (EULAR) 2021. EULAR definition of difficult to treat RA.
- Treatment according to European League against Rheumatism recommendation and failure of >2b/tsDMARDs (with different mechanisms of action) after failing csDMARD therapy (unless contraindicated).
- Signs suggestive of active progressive disease defined as >1 of :
- -At least moderate disease activity (according to validated composite measures including joint counts, for example, DAS28-ESR>3.2 or CDAI>10.
- -Signs (including acute phase reactants and imaging) or symptoms suggestive of active disease ( joint related or other).
- -Inability to taper glucocorticoid treatment below (7.5mg/day prednisone or equivalent).
- -Rapid radiographic progression (with or without signs of active disease).
- -Well controlled disease according to above standards, but still having RA symptoms that are causing a reduction in quality of life.
- -The management and signs and symptoms are perceived as problematic by rheumatologist and/or the patient
The EULAR definition of difficult to treat rheumatoid arthritis (D2T RA) considers multiple contributing factors, a high burden of disease, and the heterogeneity of D2T RA. It is a suggestion that these factors should be identified in daily practice in order to tailor therapeutic strategies further to the individual patient.
Points to Consider on Therapeutic Drug Monitoring in Inflammatory Arthritis
Charlotte Krieckaret, MD, PhD, presented a study “Points to consider for therapeutic drug monitoring of biopharmaceuticals in inflammatory RMDs” in a session at the European Alliance of Associations for Rheumatology, (EULAR) 2021. The following points were discussed :
- Measurement of biopharmaceutical blood concentrations should be performed in a validated laboratory.
- Measurement of ADAb should be performed in a validated laboratory preferably using a consistent assay over time. Measurement should be performed and interpreted along-side biopharmaceutical blood concentration.
- When interpreting bio pharmaceutical blood concentration, patient specific factors must be considered.
- There is an association between biopharmaceutical blood concentrations and clinical response
but more update is required to recommend an optimal range for most biopharmaceuticals in most indications. - Routine use of proactive TDM is not recommended in the management of inflammatory RMDs.
- Measurement of biopharmaceutical blood concentrations up to 3 months after the commencement of treatment could be considered to predict future efficacy.
- Reactive TDM could be considered in the management of inflammatory RMDs.
- Measurement of biopharmaceutical blood concentration could be considered to identify those with high biopharmaceutical blood concentrations in whom tapering may be indicated.
- Measurement of bio pharmaceutical blood concentrations should be considered to understand clinical non-response.
- Measurement of ADAb should be considered in the case of immunogenic biopharmaceuticals alongside
bio pharmaceuticals blood concentrations at the time of clinical non response. - Measurement of ADAb should be considered in the case of a hypersensitivity reaction mainly elated to infusions.
- Measurement of ADAb is to recommended in the case of an injection site reaction.
- Cost-effectiveness of TDM should be considered according to local context and standard of care.
Highlighting the potential clinical utility of the measurement of biopharmaceutical blood concentrations and ADAb and identification of factors that influence these parameters. Pro active TDM is not recommended due to lack data from clinical trials. Reactive TDM could be considered in certain clinical situations. Other factors like observational studies/post hoc analyses of RCTs and population-based data must be considered.
Recommendations for Cardiovascular Risk Management in Rheumatic and Mucoskeletal Diseases (Including SLE and Antiphospholipid
Syndrome)
Professor, Dr. MT Nurmohamad, presented a study “Recommendations for cardiovascular risk management in Rheumatic and Musculoskeletal Diseases (including SLE and the antiphospholipid syndrome)” in a session at the European Alliance of Associations for Rheumatology, (EULAR) 2021.
Clinicians should be aware of the increased CVD risk and reduction of disease activity is likely to lessen cardiovascular risk. Rheumatologists are responsible for CVD risk assessment and a management in collaboration with primary care providers, internists or cardiologists and other healthcare providers. All individuals should get regular CVD risk factor screening, risk stratification should include screening and strict control of cardiovascular risk factors. CVD risk assessment is recommended withing 6 months of diagnosis, patient education and counselling on CVD risk treatment adherence and lifestyle modification are important in the management of CVD risk.
Recommendations for SLE and APS – Lipid treatment should follow recommendations used in the general population. Patients with SLE may be candidates for prevention strategies as in the general population including low-dose aspirin based on their individual cardiovascular risk profile. Treatment with hydroxychloroquine may also reduce the risk of CVD events. A thorough assessment of traditional cardiovascular risk factors and disease related risk factors is recommended to fluid risk factor modification. In patients with SLE lower levels of blood pressure are associated with lower rates of CVD events and a blood-pressure target of <130/80mm Hg should be considered. In patients with LN, angiotensin converting enzyme inhibitors or angiotensin receptor blockers are recommended for all patients with UPCR>500 mg/g or arterial hypertension.
Recommendation for Gout, Vasculitis and other Rheumatic and Musculoskeletal Diseases – The use of cardiovascular prediction tools for the general population is recommended. For ANCA associated vasculitis, the Framingham score may underestimate the cardiovascular risk information and EUVAS model may supplement modifiable Framingham risk factors and is recommended to take into account. Blood pressure and lipid management should follow general population recommendations. Standard use of platelet inhibitors for primary prevention is not recommended. In patients with gout, diuretics should be avoided, a SUA level 0.36 mmol/L (6mg/dL) is recommended. In patients with systemic sclerosis, beta blockers should be avoided. In patients with ANCA-associated vasculitis, remission induction and remission maintenance will also reduce cardiovascular risk. In patients with GCA, an optimal glucocorticoid regimen that balances the risk of relapse and minimizes glucocorticoid use may also reduce cardiovascular risk.
Validation of existing generic and modified CVD risk prediction tools in large prospective studies and development of new disease-specific equations. Vascular imaging, circulating bio markers, identifying patient subgroups with high risk of CVD, long term effects of current and new drugs and role of antithrombotic agents in overall CVD risk in these patients. For better implementation, CVD risk awareness should be increased.
Differential Diagnosis in Suspected Axial SpA
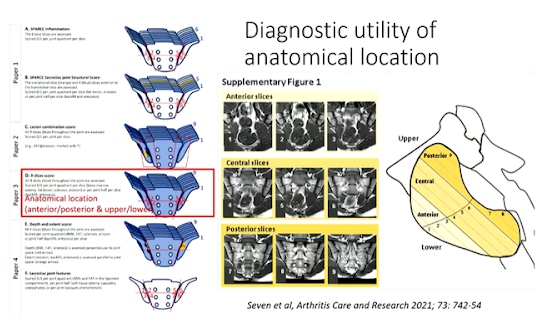
Professor Mikkel Ostergaard, presented a study “Differential diagnosis in suspected axial SpA” in a session at the European Alliance of Associations for Rheumatology, (EULAR) 2021. Both active and structural MRI lesions are considered typical of axspA. Data driven definitions for active and structural MRI lesions in the sacroiliac joint in spondyloarthritis have predictive utility. Data driven cut-offs for defining lesions on MRI which had positive predictive values (PPVs) >95% for a clinical diagnosis of axSpA
ASAS-defined BME in >3 consecutive slices or in >4SIJ quadrants or ASAS-defined erosion in>2 consecutive slices or in >3 SIJ quadrants or ASAS-defined fat lesion in >3 consecutive slices or in >5 SIJ quadrants or ASAS-defined fat lesion of >1cm depth in>2 consecutive slices
Some specific diagnoses like infections, tumours, degenerative disease, fractures, OCI, and DISH should always be considered. Sacroiliac BME is not specific as it may also be found in degenerative disease, athletes and healthy persons, and particularly post-partum women. Certain pattern of BME (high extent, large depth from articular surface, close relation to other lesion types) increase the likelihood/ specificity of being axspA as do presence of lesion combinations fat at erosion and lesions with large extent or depth. Sole presence of BME at certain locations, such as upper anterior part of joint reduces likelihood/specificity of being axspA.
Significant new & clinically relevant knowledge has been gained, but further research is still needed to optimally distinguish what is and what is not sacroiliitis.
Chronic, Low-Grade, Articular and Systemic Inflammation as a Therapeutic Target in OA
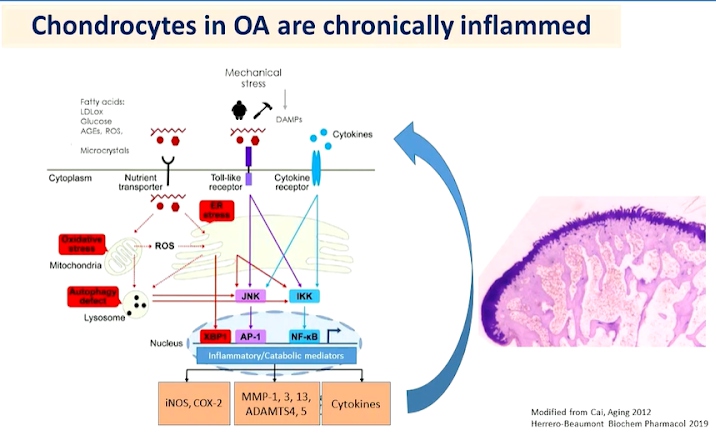
PhD Raquel Largo, presented a study “Chronic, low-grade, articular & systemic inflammation as a therapeutic target in OA” in a session at the European Alliance of Associations for Rheumatology, (EULAR) 2021. Study of the associations between Metabolic Syndrome (MetS), the number of its components and individual components like obesity are associated with incident knee Osteoarthritis (OA) but not independent of BMI and body weight. Weight and BMI are associated with hand OA development.
Low-grade chronic inflammation is not associated with increased energy expenditure. It is difficult to be detected and thus diagnosed and treated. Low-grade inflammation induced by metabolic alteration is meta-inflammation. This is the main driver of different chronic disorders like obesity. Over-nutrition is known to induce inflammation in adipose tissues. Hyperlipidaemia is also known to induce synovial inflammation.
Knee OA patients involved in a gastric surgery program with BMI 50. 7 patients showed 20% weight loss in 6 months. Benefits of weight loss on symptoms of systemic inflammation and cartilage turnover in obese patients with knee OA. However, anti-cytokine therapy was found to be ineffective for OA treatment independently by three different teams. Anti-inflammatory therapy with IL-1-therapy was found to have beneficial effects in other cardiometabolic diseases characterised by inflammation, such as diabetes, stroke, and chronic kidney disease and in patients with lung cancer. IL-1beta inhibition decreases the incidence of hip and knee replacement. Glucosamine has been used as a modulator of low-grade chronic systemic inflammation.
Low-grade chronic inflammation is a different process with the classic signs of inflammation. Induction of adipose tissue browning could decrease obesity and metabolic disorders preclinical data on IL-17A axis.

