Difficult to Treat RA: Definitions and Epidemiology
Roodenrijs N, presented a study in a session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. Outcomes of RA patients have significantly improved by B/tsDMARDs, treat-to-target and tight control strategies. Subgroups of RA patients includes: persistent signs and /or symptoms, suggestive of inflammatory RA activity and treatment with several cs/b/tsDMARDs.
D2T RA includes multifactorial and heterogenic disease, estimated prevalence 5-20% for failure of ≥2 b/ tsDMARDs. Impact on D2T RA patients includes: physical functioning, social life, work participations, etc. and on socioeconomic: health care utilisation, costs, etc. Improvement of management should be based on uniform terminology, uniform definition, more insight into contributing factors and more insight into its impact on patients-related and socioeconomic.
EULAR definition of difficult-to-treat RA includes:
- Treatment according to European League Against Rheumatism recommendation and failure of ≥2b/tsDMARDs (with different mechanisms of action)* after failing csDMARD therapy (unless contra indicated).
– Signs suggestive of active/progressive disease, defined as ≥1 of:
– At least moderate disease activity (according to validated composite measures including joint counts, for example, DAS28-ESR>3.2 or CDAI>10).
– Signs (including acute phase reactants and imaging) and/or symptoms suggestive of active disease (joint related or other).
– Inability to taper glucocorticoid treatment (below 7.5?mg/day prednisone or equivalent).
– Rapid radiographic progression (with or without signs of active disease).
– Well-controlled disease according to above standards, but still having RA symptoms that are causing a reduction in quality of life. -The management of signs and/or symptoms is perceived as problematic by the rheumatologist and/or the patient.
Concept of D2T RA has now been formalised with the EULAR definition. D2T RA is a heterogenic disease state: one or multiple contributing factors may be present. The patients & economic burden of D2T RA are significant.
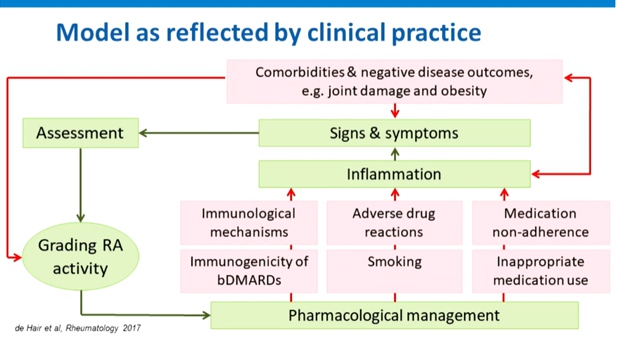
The Role of Comorbidities in Difficult to Treat RA
Nikiphorou E, presented a study in a session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. In terms of terminology there are inconsistencies in the use of terms. But nevertheless the comorbidities should be need to address one way or another. Results of an international survey reported wide variation in concepts of D2T RA & comorbidities identifies as important issues missing from current management recommendations. A U.S based study showed that multimorbidity is highly prevalent in RA as compared to without RA after diagnosis. Key messages included that comorbidity/multimorbidity are common in RA. Comorbidities may accumulate over time.
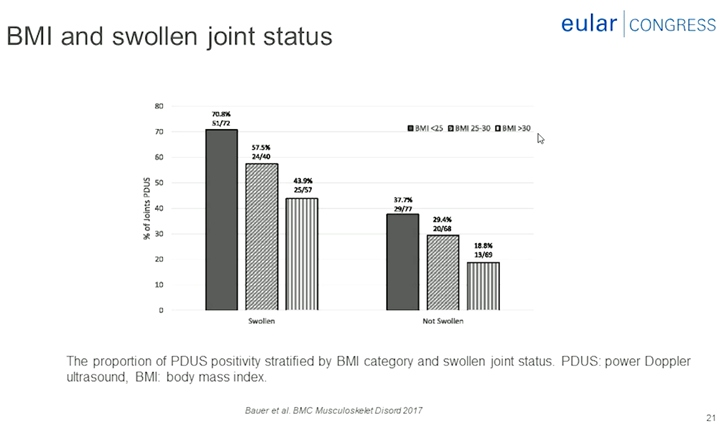
Comorbidities contribute to D2T RA can reduce the effectiveness of medication and increase adverse drug reaction & can hamper proper grading of RA disease activity may results in inappropriate treatment decisions. Obesity contribute to D2T Ra may provide a less favourable response to treatment, worse subjective measures of disease activity, increased pain and comorbidity.
Obesity contributes to D2T Ra may provide less favourable response to treatment, worse subjective measures of disease activity, increased pain and comorbidity, reduced likelihood of achieving remission in early disease, reduced probability of maintaining sustained remission by 51%, worse long-term outcome, including a higher prevalence of comorbidities for RA with obesity. Comorbidities such as obesity and fibromyalgia can complicate the assessment of disease activity. Comorbidities may negatively impact treatment goals, disease outcomes and overall prognosis. Comorbidities can influence therapeutic choice. Contributing factors and burden of disease in D2T RA includes lower socioeconomic status at RA onset, limited drug options due to adverse effects or comorbidities, mismatch in patients, and rheumatologist with to intensify treatment (37% vs. 6%) and concomitant fibromyalgia (38% vs. 9%).
Future management guidelines for RA should highlight the importance of multimorbidity and advocate appropriate screening and management. Management should be patient-centres, tailored to individual needs. Risk stratification and dedicated pathways for patients with multimorbidity and D2T RA should remain priorities on the research agenda.
What can the Synovial Tissue Learn us About Difficult to Treat RA?
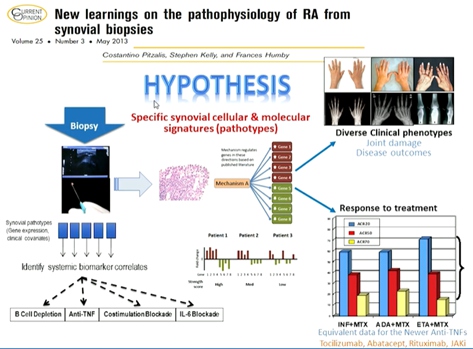
Pitzalis C, presented a study in a session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. The disease phenotypes of RA synovitis are extremely heterogeneous, with a variety of cellular and molecular signs (pathotypes) emerging as possible taxonomic classifiers. It was reported that each surgery received an average of 14 biopsy samples, with 93% of bio psy procedures yielding good quality tissue. In all joints and repeat biopsies, RNA yield was excellent. Baseline lympho-myeloid pathotype predict x-ray progression at 12 months. Baseline pauci-immune pathotype inversely correlates with treatment response.
It was hypothesised that whether the level of β-cell infiltration in the synovial tissue & related molecular pathways define treatment response to the specific β-cell depleting agent Rituximab in comparison to Tocilizumab. The Primary trail hypothesis include Tocilizumab is superior to rituximab in β-cell poor patients. Secondary trial hypothesis include is rituximab in non-inferior to Tocilizumab in β-cell rich patients.
In patients with low/absent β-cell lineage signature in synovial-tissue, Tocilizumab is significantly superior to Rituximab. Although the study was not powered for the comparative analysis of the β-cell-rich group, similar week-16 response rates was observed. Synovial tissue molecular signatures define treatment response/ non-response and refractoriness.
Synovial tissue cellular and molecular signatures define different disease pathotypes & clinical phenotypes. Fibroid/pauci-immune pathotype is associated with no response to both synthetic and biologic DMARDs. In β-cell rich patients, Rituximab and Tocilizumab appear to be as effective, though the study was not powered for non-inferiority.
Measuring Drug Levels and Anti-Drug Antibodies-what do the Data tell us and what are the Practical Consequences?
Charlotte Krieckaert, MD, PhD, presented a study “Measuring drug levels and anti-drug antibodies – what does the data tell us and what are the practical consequences?” in a session at the European Alliance of Associations for Rheumatology, (EULAR) 2021.
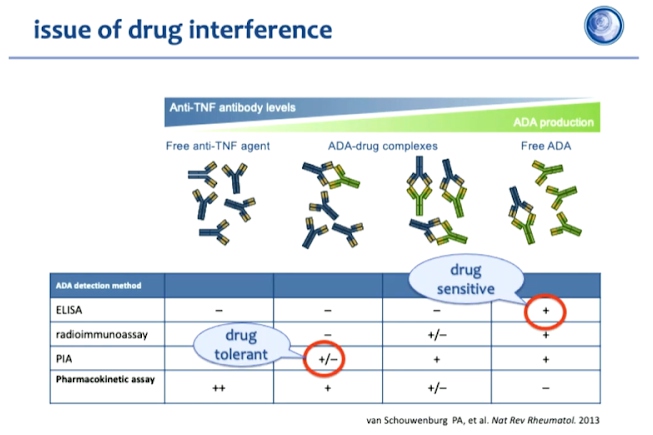
Immunogenicity is a normal response for all therapeutic anti-bodies. Therefore, biopharmaceutical concentrations in the blood must always be measured in the case of very low or absent concentration measures of ADAb. Antibody detection methods must therefore be chosen carefully to determine antidrug antibodies and respective drug levels based on the objective and kind of analysis. For clinical purposes, a drug sensitive assay, for instance, ELISA would be highly recommended. On the other hand, for scientific analysis purposes, adopting a drug tolerant assay might be considered more appropriate. However, it must be emphasised that the assays employed for any kind of analysis must be consistent over time.
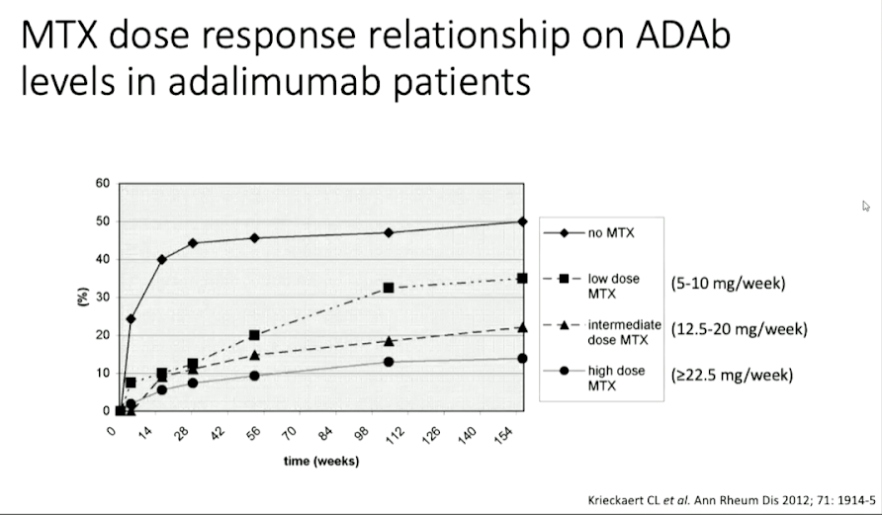
Over time anti-drug anti-bodies are developed in the body which can be detected using radioimmunoassay or pH-shi- anti- idiotype antigen binding assay. The anti-drug antibodies are neutralising and their effect is related to pharmacokinetics and methotrexate administration has been shown to suppresses immunogenicity.
Pharmacokinetics of biologicals is different as compared to traditional drugs. On a population level, biological levels are associated with the response. However, on an individual level, a larger variation is observed. Therefore, the additional value should be prospectively rested in clinical trials (not cross sectional).
Therapeutic Drug Monitoring in RA-Aspirations and Challenges
Meghna Jani, presented a study “Therapeutic drug monitoring in RA-aspirations & challenges” in a session at the European Alliance of Associations for Rheumatology, (EULAR) 2021.
Observational data demonstrates an association between higher TNFi drug levels and better 12-month treatment response. TDM may be less useful in etanercept treated patients. When interpreting drug levels, patient factors that influence PK should be considered which include BMI, methotrexate co-treatment, disease activity, and adherence to therapy. Measurement of ADAbs should be considered in the case of a hypersensitive reaction, mainly related to infusions (not injection site reactions). RA patients with high biologic drug levels may have a higher risk of all infections. However, larger prospective observational studies need to fully evaluate the association between high drug levels and serious infections.
Opportunities and challenges in the TDM landscape are numerous. TDM may be considered to identify those with high drug levels in whom tapering doses may be indicated. TDM 3 months after treatment could be considered to predict future efficacy. Conflicting evidence has been observed with regards to switching treatment strategy (to another TNFi or less immunogenic TNFi). TDM may be considered to understand clinical non-response in TNFi treated patients (especially monoclonal Abs). Proactive testing at present in not recommended based on current scientific evidence. There is a need for larger prospective studies /RCTs- tapering, comparing to standard of care, switching treatment, PK/PD parameters, and cost- effectiveness.
TDM shows promise but there is currently insufficient evidence to recommend routine adoption in rheumatoid arthritis.
Therapeutic Drug Monitoring in RA-What do the Data tell us?
Silje Watterdal Syversen, presented a study “Therapeutic drug monitoring in RA–what do the data tell us?” in a session at the European Alliance of Associations for Rheumatology, (EULAR) 2021.
Two randomised controlled trials addressing the effectiveness of proactive TDM in patients with arthritis were discussed. The NOR-DRUM trials A and B (NORwegian DRUg Monitoring trials) were performed to assess if proactive TDM improves the effectiveness of infliximab therapy in patients in patients with immune-mediated inflammatory diseases with respect to induction period (NOR-DRUM A) & maintenance therapy (NOR-DRUM B). 450 adult patients diagnosed with RA, SpA, PsA, UC, CD or Ps who were on maintenance therapy with infliximab were enrolled in the study. 50% were on TDM and rest were control. Infliximab dose 4.8mg/kg with serum infliximab level (5.5mg/L) was detected in both groups. 30% were within serum-infliximab within the window throughout and 19% with serum infliximab below the therapeutic window at least once. Proactive TDM during induction of infliximab did not lead to improvement in remission rates or in any of
the other efficacy outcomes. Also, fewer infusion reactions were observed in the TDM group.
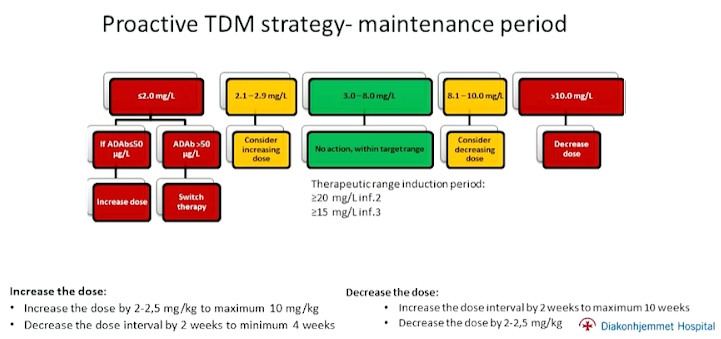
In conclusion, proactive TDM is superior to standard therapy in sustaining disease control in patients on maintenance infliximab. Clinical findings to support the same have been consistent amongst all disease sub-groups. This can allow us to achieve improved therapy without increasing overall drug consumption.
The NOR-DUM trials support implementation of proactive TDM during maintenance therapy with infliximab, but not during the induction period (with the exception of patients at high risk of immunogenicity). However, more data is needed for proactive TDM of other biologics’ and targeted TDM.
JAKi in the News-Side-Effects
Roy Fleischmann, presented a study “JAKi in the news – side effects” in a session at the European Alliance of Associations for Rheumatology, (EULAR) 2021.
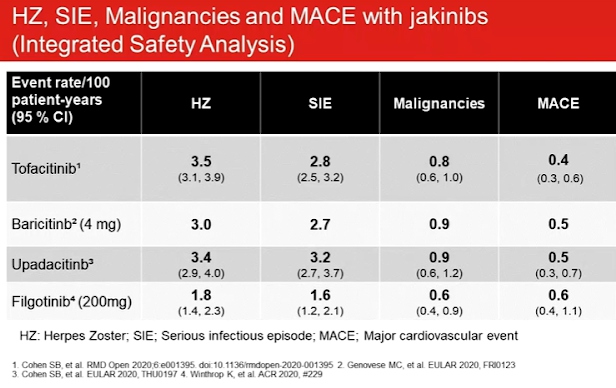
If it’s not safe, it doesn’t matter if its effective. Clinical trial data of the safety profiles for JAK inhibitors indicated malignancy (SIR by SEER database is approximately 1), increased lipids may not increase cardiovascular risk in rheumatoid arthritis & venomous thrombotic episodes. Impaired spermatogenesis and histopathological effects on male reproductive organs (testes and epididymis) were observed with filgotinib in rats and dogs. Top line results of the MANTA study of 250 males with IBD stated that 8,3% patients on placebo and 6.7% patients on filgotinib had a 50% or more decline in sperm concentration at week 13. Patients are still being followed to asses long term decline and reversibility.
Past studies have associated DMARDs with reduced risk of CV events including MXT and TNFi. As compared to TNFi, toclizumab may be associated with a reduced risk of MACE whereas csDMARDs may be associated with an increased risk of MACE and stroke. Compared to csDMARDs, mortality and CV events significantly reduced in patients treated with TNFi without increasing the risk of solid cancer in patients without or with prior malignancy by reducing chronic inflammation.
Swedish Rheumatology Quality Register linked to national registers on VTE events showed that medications like corticosteroids may be a potential co-founder and RA disease activity increases the risk of VTE in a dose dependent manner. Oral Surveillance: Pfizer study 1133, ascertained that for tofactinib the most frequently reported MACE was myocardial infarction and the most frequently reported malignancy was lung cancer.
Thrombosis, including pulmonary embolism deep vein thrombosis and arterial thrombosis have occurred in patients treated with Janus kinase inhibitors. Rheumatoid Arthritis patients with at least one CV risk factor had a higher rate of all causes mortality and thrombosis with tofactinib 10mg twice daily vs 5mg twice daily or TNF blocker.
“Selective” JAKi on the Verve-Implications for Therapy
Hendrik Schulze-Koops presented a study “Selective” JAKi on the verve – implications for therapy” in a session at the European Alliance of Associations for Rheumatology, (EULAR) 2021.
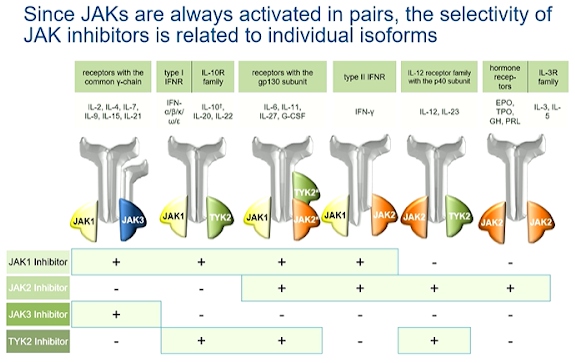
JAK inhibitors are competitive inhibitors of ATP. The Janus kinases (JAK1, JAK2, JAK3 and TYK2) always act in homo- or heterologous pairs and activate transcription via different STATs. Independent dose related effects of upadacitinib on JAK1 and JAK2 showed a plateau of JAK1 mediated efficacy at 15mg. The evidence of inhibition of JAK2 mediated haematopoiesis commenced at dose equal to or more than 15mg. Selective JAK inhibition by upadacitinib allowed for optimal dosing to maximise the efficacy while minimizing the effects mediated by other JAK isoforms.
Since JAKs are always activated in pairs, the selectivity of JAK inhibitors is related to individual isoforms. Selectivity of JAK inhibitors is relative and dose dependent. Selectivity measured in vitro for different JAK isoforms can only be projected to the clinical effects of JAK inhibitors to a limited extent. The influence on clinical parameters does not correlate necessarily with the in vivo selectivity of JAK inhibitors. The estimated IRR of severe infections compared to placebo in protocol analysis were not statistically significant.
The current data indicate that “pan-JAK inhibitors” & moderately selective JAK inhibitors are comparatively effective in RA and have a similar safety profile (laboratory parameter, herpes zoster, DVT) The concept of JAK- selectivity is based on in vitro assays with limited impact on cellular and clinical aspects of the use of JAK inhibitors.
“Ten Years” of JAKi in RA-Real Life Data from the SCQM Registry
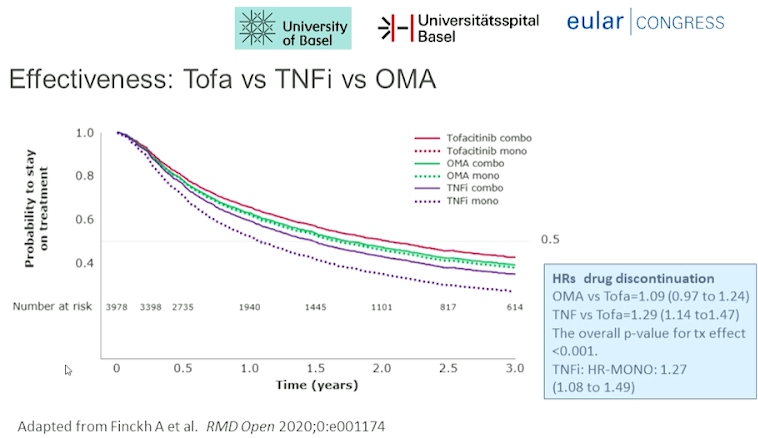
Diego Kyburz presented a study “Ten years of JAKi in RA–Real life data from the SCQM registry” in a session at the European Alliance of Associations for Rheumatology, (EULAR) 2021. The Swiss Clinical Quality Management in RA was the first registry of RA created in 1996 for the comprehensive quality management in RA.
Real world data is especially important because of its more diverse, heterogenous patient population as compared to RCTs. They help tremendously in determination of efficacy, treatment patterns and clinical decision making. They provide more detailed longitudinal information than RCTS. They also provide a comparative view with other drugs which may not be available in RCTs. Approximately 70% of Swiss Rheumatologists currently contribute to SCQM. The registry maintains population data on adult RA patients starting therapy with Tofacitinib or TNF inhibitors or other bDMARD aer August 2013. It maintains patient and disease characteristics, drug retention, safety (serious infection rate) data using methods like Kaplan Meier curves and Cox proportional hazard models.
Tofactinib (Tofa) licensed in Switzerland in August 2013, has been used to treat adult patients with moderate to severe rheumatoid arthritis with inadequate response or intolerance to methotrexate. It has been used as a monotherapy as well as in combination with conventional synthetic DMARDs. No restrictions in the prescription, equivalent to TNF- inhibitors, IL-6 antagonists or abatacept have been advised.
Tofa had higher drug retention compared to TNFi. Tofa had lower discontinuation rate for ineffectiveness than TNFi and higher discontinuation rate for intolerance than bDMARD. No difference was observed for mono- vs combination therapy in Tofa and OMA.
The Clinical Heterogeneity of Psoriatic Arthritis in Adults
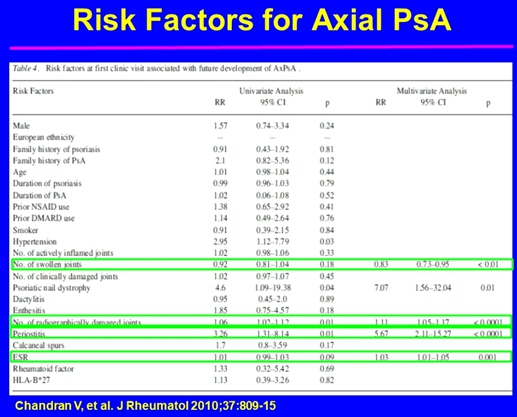
Gladman DD, presented a study in a session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. The objective of the study is to understand disease heterogeneity in PsA.
Psoriatic arthritis is an inflammatory disease associated with psoriasis. Its associated features are spondylitis, enthesitis, dactylitis, iritis, mucous membrane ulcers, urethritis and other extra-articular features of SpA. It is important to assess at least 68 joints for tenderness, 66 joints for swelling. Patients with oligoarticular presentation have a similar burden of disease as those with polyarticular presentation. It was reported that 117 of 192 oligoarticular patients (61%) remained oligoarticular and 75 (39%) progressed to polyarthritis. Lower SF-36 mental component summary score was the predictor for progressing to polyarthritis. Recurrent dactylitis was reported in 44% of the patients. Radiographic progression is more common in digits with dactylitis. Risk factors for enthesitis in those who developed aer entry to cohort presented with higher BMI, actively inflamed joints and younger age. Increased burden of inflammation over time is associated with the extent of atherosclerotic plaques in patients with psoriatic arthritis.
Psoriatic arthritis domains include peripheral arthritis, axial disease dactylitis, enthesitis, skin, and nails. MSK disease manifestations are such as peripheral arthritis, dactylitis, enthesitis and axial disease.
Towards Personalized Treatment Strategies for Psoriatic Arthritis
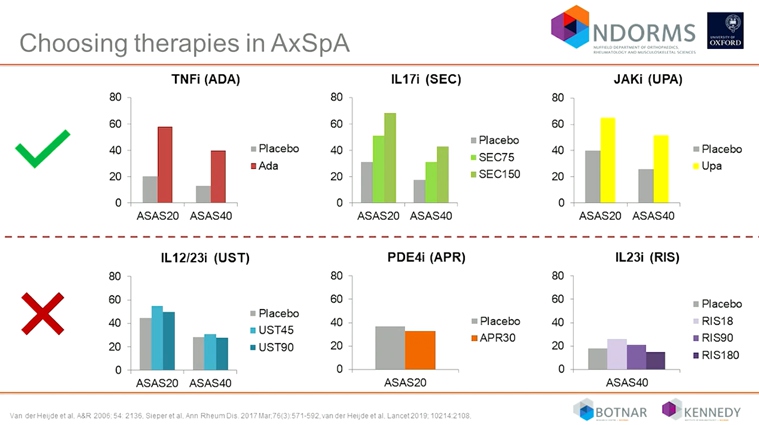
Coates L, presented a study in a session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. The EULAR 2019, GRAPPA 2015 and ACR/NPF 2018 guideline are available as the treatment recommendation used in psoriatic arthritis. The selection of the therapy should ideally be linked to the evidence based treatment for patients. The evidence predominately for the drugs that are available are csDMARDs, TNF inhibitors, IL 12/23 inhibitors, IL 17 inhibitors, T-cell costimulator, JAK inhibitors and PDE5 inhibitors.
After conducting many head to head trials, it was observed that newer therapies may be more beneficial for PsA than some of the existing biological therapies. The numbers of head-to-head trials are confirming the superiority of new biologics over TNF inhibitors or IL12/23 inhibitors. Presently TNFi and IL 17 inhibitors are approved and which has been shown beneficial in a clinical trial in AxSpA. Phase 3 trials are also reported positive results for JAK inhibitors. In contrast to this data, negative results were reported for IL 12/23 inhibitors, PDE4i and IL 23 inhibitors.
Results from randomized phase 4 control study reported the average dose of MTX was 21.8? mg/wk (55% on oral MTX) in the escalated MTX group. 85% patients received ≥ 20 mg/week, 55% received oral dose, 36% received S.C. and 9% took both. In LUNG-MAP, patients with lung SSC were assigned to sub-studies of different treatment based on their tumor biomarker profile. In ORBIT study, blood transcriptional biomarkers were identified to predict response to biologic therapy in biologic naïve patients with RA.
It is essential to know how to choose or sequence DMARDs in PsA both conventional & biological targeted drugs and also need to think beyond step up care and aim to asses prognosis more accurately in patients with more severe disease more intensively from the beginning.
Decrease in Anti-Topoisomerase-1 Antibody Titer in Patients with Systemic Sclerosis during Long-Term Rituximab Therapy
L. Garzanova, presented a study in a session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. The study’s aim is to find new option in the treatment of systemic sclerosis as there are only a few reports on the higher efficiency of RTX in patients (pts) with SSc positive for anti-topoisomerase-1 antibodies (a-Topo-1), therefore he researched on Rituximab (RTX) as a treatment.
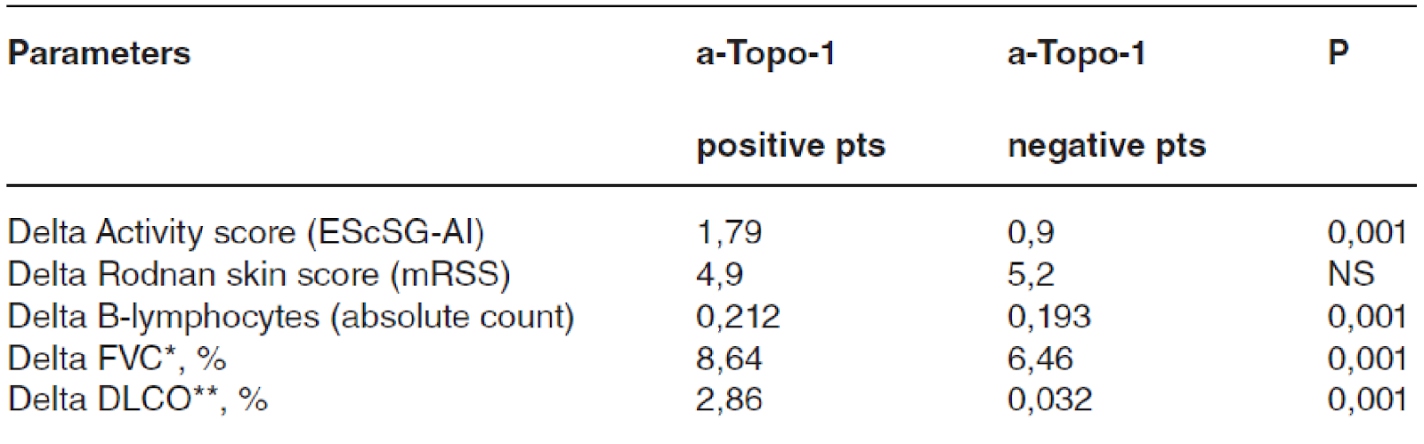
The main objective is to compare clinical parameters and B-lymphocytes (B-lymph) level in SSc pts depending on the presence or absence of a-Topo-1 during RTX therapy with prospective long-term follow -up. There were 88 people with SSc in this research. The average duration of follow-up was 26.3±10.7 months. The average age was 47 years (17-71), with females accounting for 73 points (83%) & the diffuse cutaneous subtype of the illness accounting for 50 points (57%). Interstitial lung disease (ILD) symptoms were seen in 70 of the participants (80%). The mean disease duration was 5,9±4,8 years. The cumulative mean dose of RTX was 2.9±1.1 grams. All patients received prednisone at a dose of 11.7±4.4 mg, immunosuppressant’s received 42% of them. There were 63 pts positive for a-Topo-1 and 25 pts – negative. In the a-Topo-1 positive group, 55 (87%) of the patients developed ILD, compared to just 15 (60%) in the a-Topo-1 negative group (p=0.02). The a-Topo-1 level significantly decreased during RTX therapy in Russian pts. The decrease in a-Topo-1 titers correlated with the total dose of RTX and was accompanied by a decrease in mRSS, disease activity index and an increase in FVC and DLCO.
A higher efficacy of RTX in the a-Topo-1 positive group with prevalence of ILD was revealed, therefore a-Topo-1 positivity could be considered.

