Honigberg MC. Circulation. 2021 Feb 2;143(5):410-423.
Globally, cardiovascular disease is the prime cause of death in women, and cardiovascular risk increases significantly after menopause. Premature menopause, described as menopause onset prior to age 40, is correlated with quickened biologic aging, cardiovascular disease, and all-cause mortality, with greater cardiovascular risks notified at progressively earlier menopausal age. The responsible procedures remain unclear irrespective of the well-described cardiovascular risks correlated with premature menopause. Clonal hematopoiesis of indeterminate potential (CHIP) is the expansion of hematopoietic stem cells with somatic mutations in leukemia-associated genes. CHIP is correlated with increase in inflammatory cytokines and accelerated atherosclerosis in animal and human studies. It is currently unclear if the history of premature menopause is associated with CHIP. Thus, Honigberg MC, et al., conducted a study to analyse whether premature menopause overall and distinguished by procedure of menopause (natural and surgical premature menopause) was correlated with increased risk of CHIP between postmenopausal women in the UK Biobank and Women’s Health Initiative (WHI). The correlation of CHIP with incident coronary artery disease (CAD) events was evaluated in these groups.
11,495 postmenopausal women from the UK Biobank aged 40-70 years with whole exome sequences and 8,111 women from Women’s Health Initiative aged 50-79 years with whole genome sequences were included in the study. Mean (SD) age at blood draw for whole exome and whole genome sequencing was 60.1 (5.2) years in the UK Biobank and 68.3 (6.6) years in WHI, respectively. The WHI group incorporated 6,733 (83.0%) White, 918 (11.3%) Black, 217 (2.7%) Hispanic, and 157 (1.9%) Asian/Pacific Islander women. Premature menopause was described as natural or surgical menopause arising before age 40 years. Co-primary outcomes were the presence of (1) any CHIP and (2) CHIP with variant allele frequency (VAF) >0.1. The correlation of premature menopause with CHIP was analysed using logistic regression, adjusted for age, race, the first 10 principal components of ancestry, smoking, diabetes mellitus, and hormone therapy use. Secondary analyses considered natural vs. surgical premature menopause and gene-specific CHIP subtypes. The correlation among CHIP and incident CAD was analysed using multivariable-adjusted Cox models.
In the UK Biobank, 390 women (3.4%) showed a history of premature menopause, of whom 178 women (1.5%) showed natural premature menopause and 212 women (1.8%) showed surgical premature menopause (46 [0.4%] with bilateral oophorectomy ± hysterectomy vs. 166 [1.4%] with hysterectomy alone). In WHI, 915 women (11.3%) showed a history of premature menopause, of whom 240 women (3.0%) showed natural premature menopause and 675 women (8.3%) showed surgical premature menopause (511 [6.3%] with bilateral oophorectomy ± hysterectomy vs. 164 [2.0%] with hysterectomy alone).
Consistent with the older age of women enlisted and higher number of CHIP drivers determined in WHI, CHIP prevalence was higher in WHI as compared to the UK Biobank: 698 (8.6%) vs. 415 (3.6%) (p<0.001). With older chronologic age, CHIP prevalence elevated progressively. CHIP prevalence was greater in women with vs. without premature menopause irrespective of age at blood draw; differences in CHIP prevalence were more pronounced for with CHIP with VAF >0.1. (8.8% (115/1,305) vs. 5.5% (998/18,301)) (Figure 1).
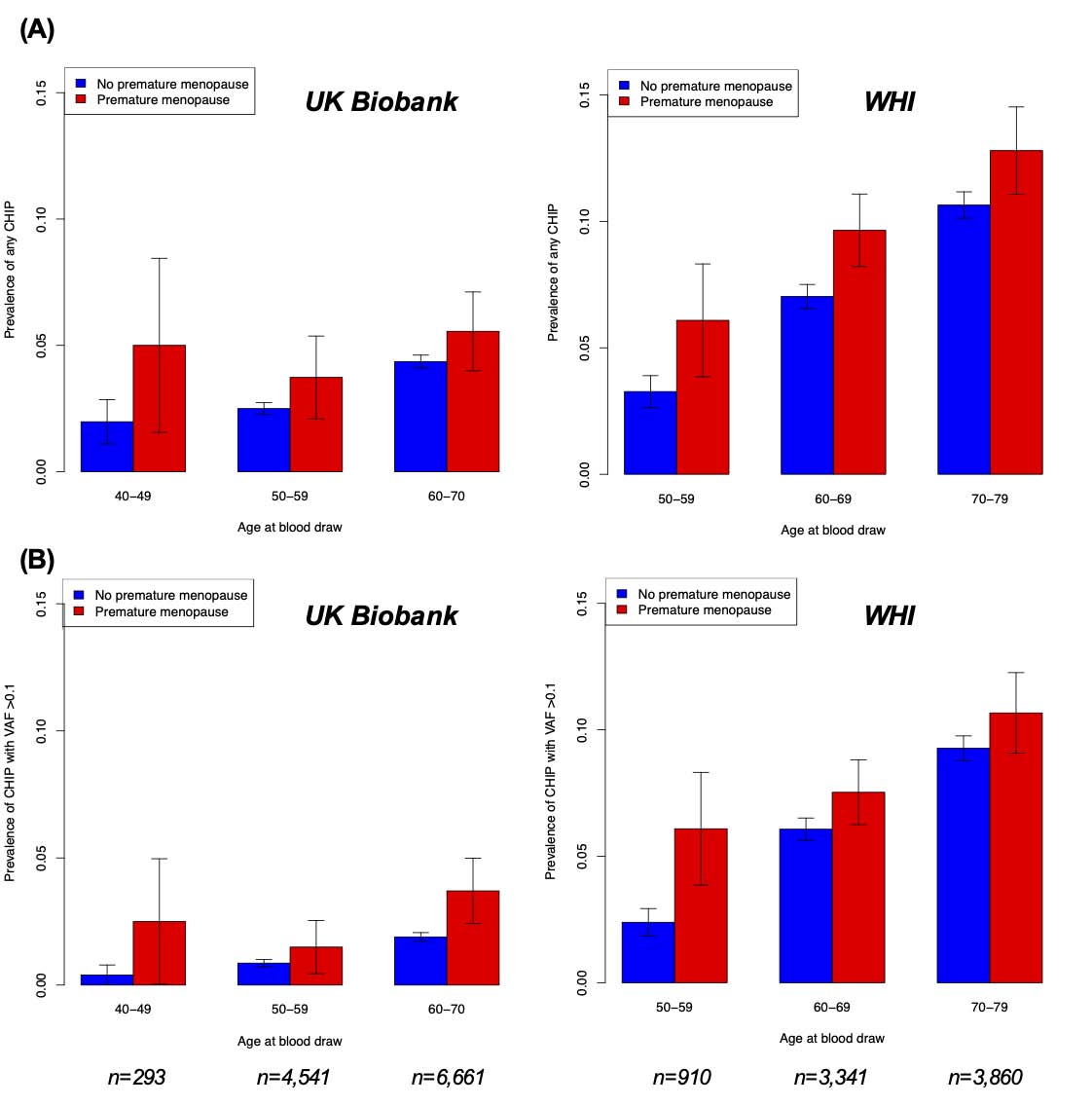
Figure 1: Prevalence of (A) any CHIP and (B) CHIP with variant allele frequency >0.1 by age and premature menopause status in the UK Biobank and Women’s Health Initiative. Error bars represent ±1 standard error of the sample proportion
Premature menopause was correlated with all CHIP with an odds ratio (OR) of 1.35 (95% CI 0.83-2.17, p=0.22) in the UK Biobank and an OR of 1.36 (95% CI 1.07-1.73, p=0.01) in WHI, bringing a meta-analyzed OR of 1.36 (95% CI 1.10- 1.68, p=0.004; p(heterogeneity)=0.97) in our primary analysis. A higher correlation among natural premature menopause and CHIP mainly drives this correlation (meta-analyzed OR 1.73, 95% CI 1.23-2.44, p=0.001; p(heterogeneity)=0.86), than observed relationship among surgical premature menopause and CHIP (meta-analyzed OR 1.21 95% CI 0.93-1.57, p=0.15; p(heterogeneity)=0.81). In CHIP with VAF >0.1, correlation with natural premature menopause occurred even higher (UK Biobank: OR 2.72, 95% CI 1.17- 6.29, p=0.02; WHI: OR 1.75, 95% CI 1.13-2.69, p=0.01; meta-analyzed OR 1.91, 95% CI 1.30- 2.80. p<0.001; p(heterogeneity)=0.26), whereas there was no evidence of correlation with surgical premature menopause (Figure 2).
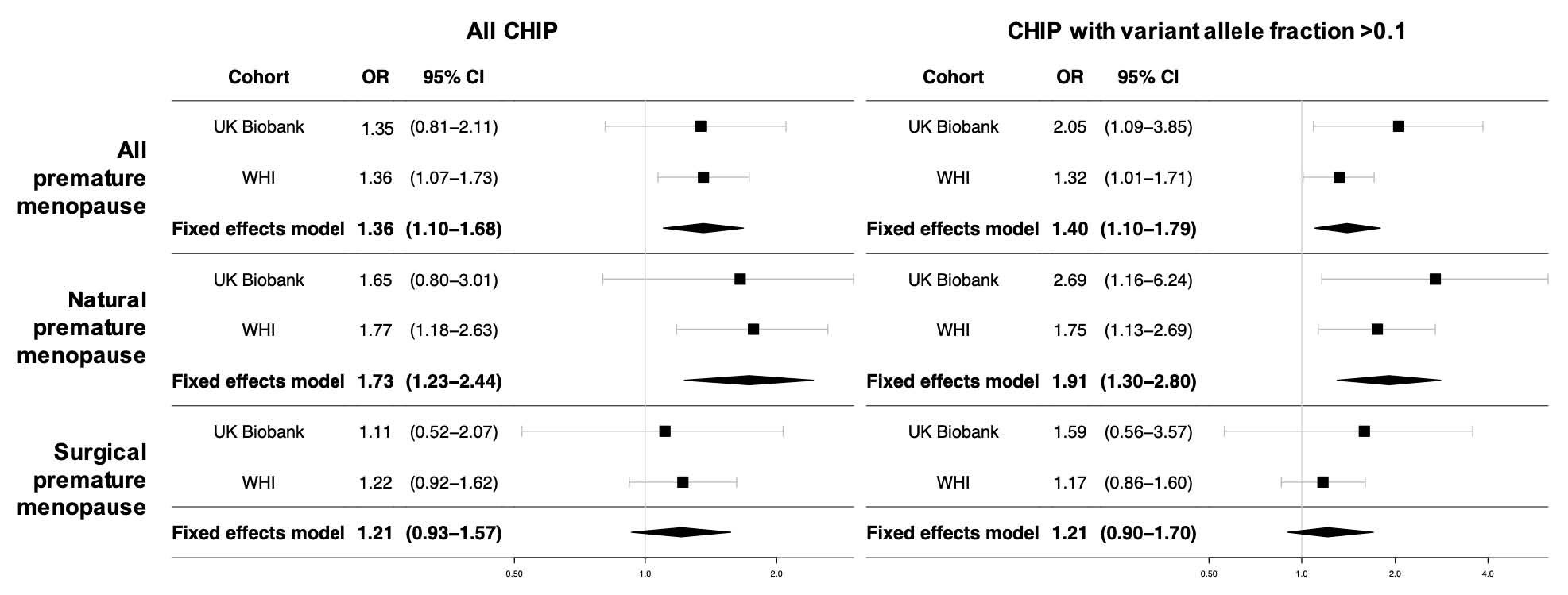
Figure 2: Meta-analysis of associations between premature menopause and CHIP in the UK Biobank and Women’s Health Initiative. WHI models are further adjusted for race/ethnicity, enrollment in the WHI observational study vs. clinical trial, and whether women were randomized to hormone therapy vs. placebo.
In gene-specific analyses, only DNMT3A CHIP was substantially correlated with premature menopause. CHIP was individualistically correlated with incident coronary artery disease in postmenopausal middle-aged women, (HR associated with all CHIP: 1.36, 95% CI 1.07-1.73, p=0.012; HR associated with CHIP with VAF >0.1: 1.48, 95% CI 1.13-1.94, p=0.005) (Figure 3).
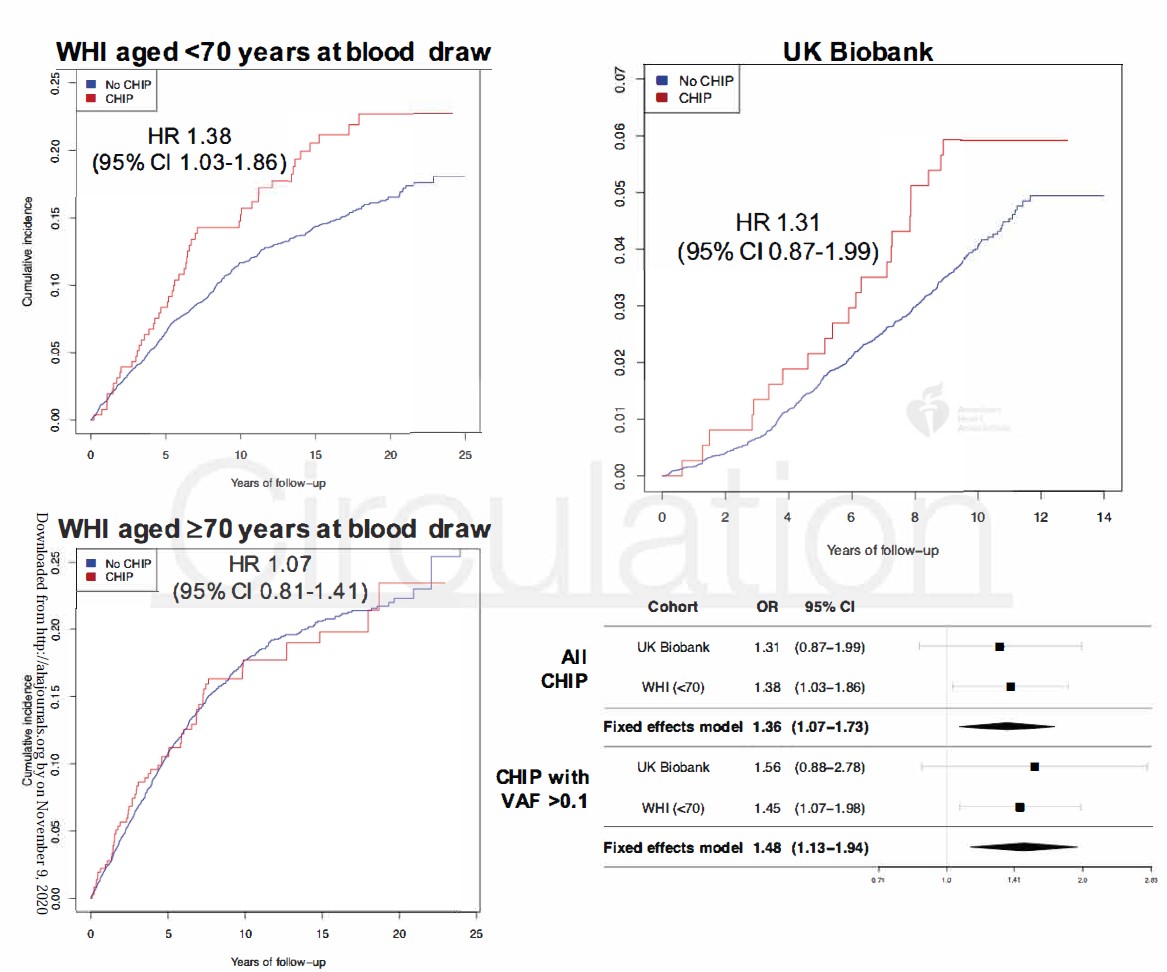
Figure 3: Incidence of coronary artery disease in women with and without CHIP in the UK Biobank and Women’s Health Initiative. Cox proportional hazard models are adjusted for age, the first 10 principal components of ancestry, current or former tobacco use, prevalent diabetes mellitus, systolic blood pressure, antihypertensive medication use, cholesterol-lowering medication use, body-mass index, prior hysterectomy, and a history of prior hormone therapy use; WHI models are further adjusted for race/ethnicity, enrollment in the WHI observational study vs. clinical trial, whether women were randomized to hormone therapy vs. placebo, and a WHI inverse probability weight.
Thus, it was concluded that in postmenopausal women, premature menopause, primarily natural premature menopause, is independently correlated with CHIP. CHIP may lead to excess cardiovascular risk correlated with premature menopause. These findings should trigger additional research to explain procedures of CHIP progression in this population.

