SCOPE II: A Randomized Trial of Two Self-expanding TAVR Bioprostheses
Tamburino C, presented findings of the SCOPE II trial at the TCT Connect 2020 virtual conference. The SCOPE II trial was designed to compare the clinical outcomes of the ACURATE neo and CoreValve Evolut valves. A total of 796 patients aged 75 years or older with symptomatic severe aortic stenosis undergoing TAVR as established by the Heart Team were recruited at 23 tertiary heart valves centers in Denmark, France, Germany, Italy, Spain and the United Kingdom. Participants were randomly assigned (1:1) to receive treatment with the ACURATE neo (n=398) or the CoreValve Evolut devices (n=398). The primary endpoint (non-inferiority) was all-cause death or stroke at 1 year. The key secondary endpoint (superiority) was new permanent pacemaker implantation at 30 days. In the intention-to-treat analysis, death or stroke at 1 year occurred in 15.8% patients in the ACURATE neo group compared to 13.9% in the CoreValve Evolut group, while in the per-protocol analysis it was 15.3% vs. 14.3%. In the intent-to-treat analysis, non-inferiority of the ACURATE neo was not met for the primary endpoint, while it was met in the per-protocol analysis. Depending on the prespecified statistical plan, non-inferiority was not defined for the primary endpoint. With ACURATE neo, new pacemaker implantation at 30 days was 10.5% compared to 18.0% with CoreValve Evolut (risk difference -7.5%, 95% CI -12.4 2.60, p = 0.0027). In the ACURATE neo group, cardiac death was higher at 30 days (2.8% vs. 0.8%, p = 0.03) and one year (8.4% vs. 3.9%, p=0.01) The moderate-severe aortic regurgitation rate was increased in the ACCURATE neo group 9.6% vs. 2.9% (p<0.0001) at 30 days and 4.0% vs. 3.3% (p<0.0001) at one year.
Conclusion: Compared to the CoreValve Evolut bioprosthesis, TAVR with the ACURATE neo valve did not fulfil non-inferiority with respect to a composite of 1-year death or stroke. In a secondary study with minimal statistical power, in patients receiving the ACURATE neo valve, cardiac mortality increased at 1 year of age. With regard to technological characteristics, such as the degree of aortic regurgitation and the need for new permanent pacemaker implantation, the two bioprostheses differed.

REFLECT II: A Randomized Trial of a Cerebral Embolic Protection Device During TAVR
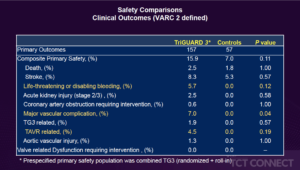
Moses JW, presented findings of the REFLECT II trial at the TCT Connect 2020 virtual conference. The REFLECT II trial tested the safety and efficacy of the self-stabilizing cerebral embolic deflection filter TriGuard 3 (TG3) in patients undergoing TAVR. REFLECT II was intended to randomise 295 patients 2:1 to TAVR with TG3 vs. control. A composite of all-cause death, stroke, life threatening or disabling bleeding, stage 2/3 acute kidney injury, coronary artery obstruction requiring intervention, significant vascular complication, and valve-related dysfunction requiring intervention (VARC 2 defined) at 30 days was the primary safety endpoint. The endpoint was compared with a Performance Goal (PG) of 34.4%. A hierarchical composite of all-cause mortality or stroke at 30 days, NIHSS deterioration, absence of post-procedure diffusion-weighted magnetic resonance imaging (DWI) lesions, and total volume of cerebral lesions (TLV) by DWI were the primary efficacy endpoints. For each patient, cumulative scores obtained using the Finkelstein-Schoenfeld method were summarised and compared between groups. The REFLECT II analysis population included 283 patients [41 roll-in, 121 randomized TG3 and 121 controls (58 randomized phase 2 and 63 pooled from REFLECT phase 1)]. In 100% of cases, TG3 was delivered and placed in the aortic arch prior to TAVR and was recovered intact in all cases. The primary safety endpoint (PG 21.3 % vs. 34 % p<0.001) was reached by TG3. The primary efficacy endpoint superiority was not met, with similar win-ratios and win % between groups (TG3 0.84 (45.7 %) vs. 1.19 (54.3 %), p=0.85). With TG3 protection, the median TLV was not different (215mm3 vs. 188mm3). Among randomized and RI patients treated with TG, 89/150 (59.3%) had full cerebral coverage (FC); those with FC and brain MRI (N=62) had a numerical 23% reduction in TLV (145mm3 vs. 188mm3). A post-hoc multi-threshold lesion-wise analysis investigated per-subject TLV above incremental thresholds from > 100mm3 to > 1000mm3 per subject TLV and showed numerical reductions with TG compared to controls for TLVs > 500mm3 (-9.7%) and > 1000mm3 (-44.5%). Among patients with full coverage (53.4%), these reductions were more pronounced: >500mm3 (-51.1%) and >1000mm3 (-82.9%).
Conclusion: Compared with historical controls, TG3 cerebral protection during TAVR was safe, however, the primary efficacy endpoint was not reached. A multi-threshold post hoc MRI study suggests the advantage of TG3 is the avoidance of larger ischemic brain lesions.
MITHRAS: A Randomized Trial of ASD Closure After TMVr
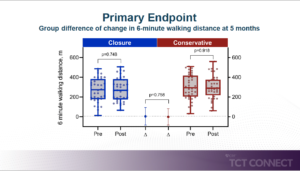
Lurz P, presented findings of the MITHRAS trial at the TCT Connect 2020 virtual conference. MITHRAS trial investigated whether the closure of a Transcatheter mitral valve repair (TMVR) induced iatrogenic atrial septal defect (iASD) is superior to medical therapy alone. In this prospective randomized single-center trial, patients with a persistent iASD one month following TMVR and relevant left-to-right shunting (Qp:Qs ³1.3) were randomly allocated to standard medical therapy alone or closure of the iASD using the Occlutech ASD occluder in a 1:1 ratio. The primary endpoint was 6-minute walk distance change 5 months after iASD occlusion; secondary endpoints included all-cause mortality and 1-year hospitalisation rate of heart failure. Moreover, the outcomes of the two randomised treatment groups will be compared with those of the contemporary cohort of patients in whom no relevant iASD occurred one month after TMVR. A total of 80 patients were enrolled between 01/2016 and 10/2019. All patients underwent TMVR using the MitraClip device 1 months prior to enrolment and were diagnosed with an iASD with relevant left-to-right shunting on transesophageal echocardiography. In total, 40 patients were randomised to the iASD closure group and 40 patients were randomised to the medical therapy group alone. Similar baseline characteristics were found between groups (mean age 77±9 vs. 76±10, years; median EuroSCORE II 4.9 (IQR 3.3-9.6) vs. 5.5 (2.6-7.6) %, left ventricular ejection fraction 38±13 vs. 37±19 %, residual severe mitral regurgitation (n=1, 3%) vs. 1 (3%), transmitral valve mean gradient 4.2±1.4 vs. 3.8±1.8 mmHg, Qp: Qs 1.5 (IQR 1.4-1.6 vs. 1.5 (IQR 1.3-1.6), severe tricuspid regurgitation (n=21, 52% vs n=18, 45%). In all 40 patients, iASD closure was successfully performed (mean occluder size 14 [IQR 12-16] mm). The 1-month post-transcatheter mitral valve repair interventional closure of iASD was not superior to conservative treatment with respect to the primary endpoint 6-minute walking distance. No difference in secondary endpoints, such as signs of heart failure or hospitalisation and survival, verifies the findings.
Conclusion: The involvement of iASD, irrespective of its administration, is correlated with a higher rate of HF hospitalisation compared to patients without relevant iASD following TMVR. Following transcatheter mitral valve procedures, the presence of an iASD could be a prognostically significant surrogate, but not inherently causative for inferior performance.
Disrupt CAD III: Safety and Effectiveness of Intravascular Lithotripsy for Treatment of Severe Coronary Calcification
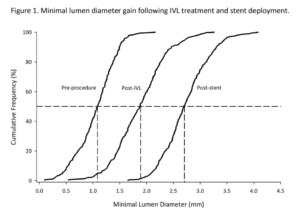
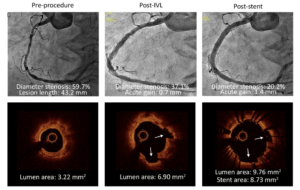
Kereiakes DJ, presented findings of the Disrupt CAD III trial at the TCT Connect 2020 virtual conference. Coronary calcification hinders the delivery and expansion of stents and is related to adverse outcomes. Intravascular lithotripsy (IVL) provides waves of acoustic pressure to adjust calcium, enhance vessel compliance, and maximise the application of stents. The objective of this trial was to assess the safety and efficacy of IVL in de novo coronary lesions that are heavily calcified. Disrupt CAD III was a prospective, single-arm multicenter study designed for regulatory approval of coronary IVL. The primary safety endpoint was the 30-day freedom from MACE (composite of cardiac death, MI or revascularization of target vessels). The primary efficacy endpoint was procedural performance, defined by QCA without in-hospital MACE as successful stent delivery with a residual stenosis of < 50 %. A pre-specified performance goal (PG) was compared to both endpoints. To test the mechanisms for IVL calcium alteration, an optical coherence tomography (OCT) sub-study was conducted. At 47 sites in four nations, patients (n=431) were enrolled. The primary safety endpoint of the 30-day freedom from MACE was 92.2%; 89.5 % was the lower bound of the 95 % CI, which surpassed the PG of 84.4 % (p<0.0001). 92.4 % was the primary efficacy endpoint of procedural success; 90.2 % was the lower bound of the 95 % CI, which surpassed 83.4 % PG (p<0.0001). The mean length of the calcified segment was 47.9±18.8 mm, the calcium angle was 292.5±76.5 °, and the calcium thickness at the maximum calcification site was 0.96±0.25 mm. In 67.4 % of lesions, OCT showed multi-plane and longitudinal calcium fractures after IVL. The minimum stent area was 6.5 ± 2.1mm2 and was comparable, independent of demonstrable OCT fractures.
Conclusion: Disrupt CAD III has demonstrated the protection and efficacy of IVL to promote stent delivery and maximise stent expansion in severely calcified CAD. With low rates of major peri-procedural clinical and angiographic complications, IVL prior to DES implantation was well tolerated.
XIENCE 90/28: Assessment of Three-Month and One-Month DAPT After Everolimus-Eluting Stents in High-Bleeding Risk Patients
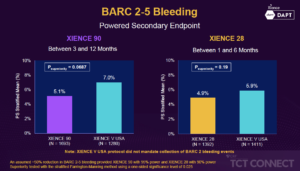
Mehran R, presented findings of the XIENCE 90/28 trial at the TCT Connect 2020 virtual conference. The XIENCE 90 trial (NCT03218787) is a prospective, single-arm, multi-center, open-label trial performed in the United States to determine the safety of 3-month DAPT in HBR patients with XIENCE everolimus-eluting stent undergoing PCI. Three months of DAPT were administered to enrolled patients after active PCI. The “3-month clear” was described as those who were free of ischemic events and adhered to the antiplatelet regimen within the first 3 months. Clear subjects were entitled to receive 3 to 12 months of antiplatelet monotherapy (aspirin) medication. The primary endpoint of the analysis is the composite rate of all-cause death or any myocardial infarction from 3 to 12 months in the “3-month clear” population. The post-approval study of XIENCE V USA will act as historical control through a stratified review of the propensity ranking. Bleeding Academic Research Consortium (BARC) type 2-5 bleeding and definite/probable stent thrombosis from 3 to 12 month are designed as major secondary endpoints. A total of 2,047 HBR subjects were enrolled in XIENCE 90 across 106 US sites. XIENCE 90 demonstrated similar rates of all death or MI between three and 12 months in the test group versus the control (5.4% vs. 5.4%, one-sided 97.5% UCL: 2.23%, pnon-inferiority=0.0063). XIENCE 28 also demonstrated non-inferiority of death or MI in the test group between one and six months (3.5% vs. 4.3%, one-sided 97.5% UCL: 0.97%, pnon-inferiority=0.0005). For the secondary endpoint, the incidence of bleeding (BARC 2-5) was 5.1% vs. 7.0% (psuperiority=0.0687 in XIENCE 90 and 4.9% vs. 5.9% (psuperiority=0.19) in XIENCE 28. Major bleeding (BARC 3-5) was significantly reduced in both trials (XIENCE 90: 2.2% vs. 6.3%, psuperiority<0.0001; XIENCE 28: 2.2% vs. 4.5%, psuperiority=0.0156).
Conclusion: Among HBR patients undergoing PCI with the XIENCE stent, a short DAPT regimen of 1 or 3 months compared with standard DAPT upto 12 months resulted in: non-inferior ischemic outcomes, similar rates of clinically relevant (BARC 2-5) bleeding, a significant reduction in major (BARC 3-5) bleeding with very low incidence of stent thrombosis.
DEFINE-PCI: One-Year Outcomes in Patients With Post-PCI Residual Ischemia
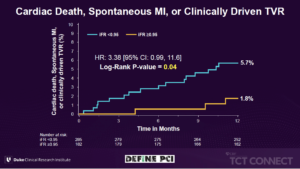
Patel MR, presented findings of the DEFINE- PCI trial at the TCT Connect 2020 virtual conference. The DEFINE-PCI trial was designed to assess the change in the Seattle Angina Questionnaire Angina Frequency (SAQ-AF) score during 1-year follow-up, to assess clinical events (CV death, MI, and target vessel revascularization) at 1-year and perform post-hoc analysis to determine if there is a target post-PCI instant wave-free ratio (iFR) value associated with improved outcomes. The DEFINE PCI was a multicenter, prospective, observational study in which a blinded iFR pullback was performed after angiographically successful PCI in 500 patients. Patients were followed 1-year for clinical events and blindly adjudicated. The primary endpoint included rate of residual ischemia defined as iFR <0.90 after operator-assessed angiographically successful PCI. The secondary endpoints included clinical events (cardiac death, MI, and target vessel revascularization [TVR]) at 1-year and change in the Seattle Angina Questionnaire angina frequency (SAQ-AF) score during follow-up. Post-hoc analysis identified achieving a post-PCI iFR value ≥0.95 to optimally discriminate clinical events. Residual ischemia (iFR<0.90) after angiographically successful PCI was seen in 24% of patients, 81.6% of which was attributable to angiographically unapparent focal lesions. 63.1% of patients with a post-PCI iFR <0.90 had an increase in SAQ-AF of ≥ 10 points at 12 months from the baseline value of 73.3 ± 22.8 vs. 71.0% of patients with iFR ≥ 0.90 (p=0.17). SAQ-AF increased ≥ 10 points more frequently in patients with compared with those without a post-PCI iFR ≥ 0.95 (100.0% vs. 88.5%, p=0.01) in majority of symptomatic patients with baseline SAQ ≤60. A post-PCI iFR ≥ 0.95 was associated with a significant reduction in cardiac death, spontaneous MI, or clinically driven TVR compared with a post-PCI iFR <0.95 (1.8% vs. 5.7%, respectively; p=0.04).
Conclusion: Patients who were highly symptomatic at baseline despite angiographically successful PCI, without residual ischemia by post-PCI iFR (iFR ≥0.95) tended to have greater improvements in anginal symptoms at 12 months compared with patients with residual ischemia. The clinical effectiveness of iFR guidance (target iFR ≥0.95) to identify and abolish post-PCI ischemia will be studied in the prospective randomized DEFINE-GPS trial.
ULTIMATE: Three-Year Outcomes After IVUS-Guided vs Angiography Guided DES Implantation
Zhang J, presented findings of the ULTIMATE trial at the TCT Connect 2020 virtual conference. For complex coronary lesions undergoing DES implantation, IVUS guidance has been recommended. In randomised trials, the long-term impact of IVUS guidance beyond 2 years in the modern DES era has scarcely been published. In comparison with angiography guidance, the multi-center randomised ULTIMATE trial showed less 1-year TVF after IVUS-guided DES implantation for all-comers.
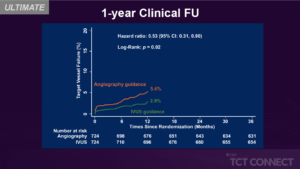
This study records the ULTIMATE trial’s 3-year clinical outcome. A total of 1448 all-comers who were randomly assigned to either IVUS guidance or angiography guidance in the ULTIMATE trial undergoing DES implantation were monitored for 3 years. The primary endpoint was the 3-year TVF risk. The safety endpoint was a definite or probable ST. If all IVUS conditions were met, the index PCI was described as the optimal procedure. In 47 (6.5 %) patients in the IVUS guidance group and in 76 (10.5 %) patients in the angiography guidance group, TVF occurred at 3 years (p = 0.01), guided primarily by decreased clinically guided target vessel revascularization (4.4 % vs. 6.8 %, p = 0.05). In the IVUS guidance group, definite or likely ST rates were 0.1 % and in the angiography guidance group, 1.1 % (p = 0.02). Notably, the optimal procedure described by IVUS was associated with a significant reduction in 3-year TVF compared to the suboptimal procedure (4.2% vs. 9.1%, p=0.01).
Conclusion: During the 3-year follow-up period, IVUS-guided DES implantation was associated with significantly lower rates of TVF and ST in all patients, particularly in patients with IVUS-defined optimal procedures, compared to angiography guidance.
Cameo Registry – Cangrelor in Acute Myocardial Infarction: Effectiveness and Outcomes Registry
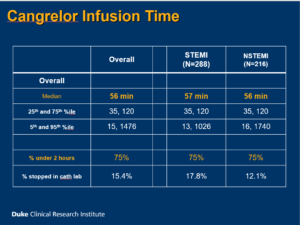
Rymer J, presented a study at TCT Connect 2020 virtual conference which analysed the timing and patterns of cangrelor use related to coronary angiography in MI patients and duration of cangrelor use was examined in MI patients undergoing coronary angiography. 3000 patients were enrolled from 10 hospitals in which 50 patients were given consecutive treatment in phase 1 and 2500 patients were given cangrelor/non-cangrelor treatment in 2:1 ratio in phase 2. In phase 1, patients were ≥ 18 years of age. Coronary angiography was done for STEMI or NSTEMI and either cangrelor or oral P2Y12 inhibitors were received during first 48 hours of MI hospitalization. In phase 2, patients with STEMI were given opiate within 24 hours prior to primary PCI and P2Y12 inhibitor was received within 7 days prior to CABG; patients with NSTEMI were given any 2 without prior PCI or CABG who had: age>60 years, male sex, diabetes, LVEF <40% and P2Y12 inhibitor was received within 7 days prior to CABG. Finally, 504 patients were treated with cangrelor, while in phase 1 (consecutive MI patients) 198/336 (58.9%) received cangrelor (53.0% STEMI, 47.0% NSTEMI). The overall median infusion time of cangrelor was 56 mins and overall percentage of cangrelor under 2 hours was 75% for both STEMI and NSTEMI patients. Cangrelor initiation time relative to Start of PCI was 15 min and 25 min in STEMI and NSTEMI patient’s respectively. In 22.7% patients with STEMI and 8.7% patients with NSTEMI, cangrelor was started before PCI. In 77.3% patients with STEMI and 91.3% patients with NSTEMI, cangrelor was started during PCI. Among patients who started oral P2Y12 inhibitor after cangrelor (excluding patients receiving oral P2Y12 inhibitors before cangrelor), the median duration of cangrelor infusion was 56 min, 43 min and 143 min in ticagrelor, prasugrel and clopidogrel group respectively. In clopidogrel group, 1222 patients overlapped with cangrelor and 71.4% patients after > 1 hour gap. In 93%, 83.3% and 36% patients were given loading dose in ticagrelor, prasugrel and clopidogrel group respectively.
Conclusion: Both patients with NSTEMI and STEMI had routine use of cangrelor at U.S. centers. Majority of patients with MI acquired less than a 2-hour infusion of cangrelor. Many patients treated with cangrelor showed a delay before loading with an oral P2Y12 inhibitor. To enhance use of cangrelor in the care of patients with MI, opportunities are existing.
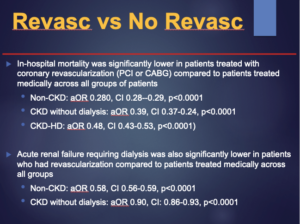
Impact of Chronic Kidney Disease on Coronary Revascularization and In-Hospital Outcomes in Patients with Acute ST-Segment Elevation Myocardial Infarction
Panchal HB, presented a study at TCT Connect 2020 virtual conference which evaluated the impact of CKD on guideline directed coronary revascularization and outcomes between patients with STEMI. This study was retrospective cross-sectional study in which the Nationwide Inpatient Sample dataset from 2012-2014 was used to recognize all hospitalized patients with STEMI. Patients were classified as non-CKD, CKD without dialysis, and CKD with dialysis (CKD-HD). Primary outcomes were guideline directed revascularization, death and acute renal failure requiring dialysis. Outcomes were assessed using hierarchical multivariable logistic regression models and p<0.05 was considered as the level of substantial. The guideline directed interventional or revascularization outcomes were coronary angiography, PCI, use of thrombolytic agents, coronary artery bypass grafting (CABG), use of intra-aortic balloon pump (IABP) and percutaneous mechanical circulatory assist devices as appropriate. The clinical outcomes were in-hospital mortality, acute renal failure requiring dialysis and other post-procedural complications. Average LOS and hospital cost of care were also compared among groups. The NIS database contains cost of hospitalization, which was adjusted for inflation as per the US consumer price index. 107,250 patients with STEMI aged 18 or older were enrolled in the study out of which 180 patients with missing data (0.17%) were excluded. Patients treated with coronary revascularization showed substantially lower in-hospital mortality (PCI or CABG) than patients treated medically over all groups of patients i.e. Non-CKD: aOR 0.280, CI 0.28–0.29, p<0.0001; CKD without dialysis: aOR 0.39, CI 0.37-0.24, p<0.0001; CKD-HD: aOR 0.48, CI 0.43-0.53, p<0.0001. Also, patients who had revascularization showed substantially lower acute renal failure requiring dialysis than patients treated medically across all groups i.e. Non-CKD: aOR 0.58, CI 0.56-0.59, p<0.0001; CKD without dialysis: aOR 0.90, CI: 0.86-0.93, p<0.0001. Significant predictors of in-hospital mortality with OR > 1.5 were CHF, liver disease and cirrhosis, PVD. The predictors of prolonged LOS were anemia because of blood loss (OR 3.75), CHF (OR 10.14), anemia because of deficiency (OR 3.4), CABG (OR 37.68), atrial fibrillation/flutter (OR 2.61), chronic lung disease, liver disease and cirrhosis and PVD.
Conclusion: Patients with CKD who present with STEMI have substantially less PCI than patients without CKD. Coronary revascularization for STEMI in CKD patients is correlated with lower mortality as compared to medical management. The presence of CKD in patients with STEMI is correlated with worse outcomes including higher mortality, acute renal failure needing dialysis, prolonged hospital stay and greater hospital cost.
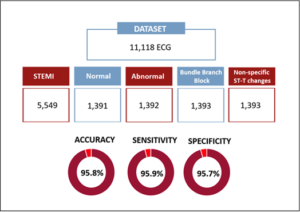
Frontline Strategies in STEMI Management: Creating Artificial Intelligence Algorithms for EKG Diagnosis
Worldwide, there is a shortage of cardiologists straight away available to explain ECG. Current software is troubled with misdiagnosis. To cope up these challenges, Mehta S, presented a study at TCT Connect 2020 virtual conference, which explored Artificial Intelligence strategies to raise novel algorithms for immediate and precise STEMI diagnosis. A dataset of 11,118 was classified as anonymous ECG from the cardiologist-annotated repository. Ontology organized 5 groups allocated for an interclass balance between common STEMI differential diagnoses; Dataset: 11,118 ECG (5,549 STEMI; 1,391 Normal; 1,392 Abnormal; 1,393 Bundle Branch Block; and 1,393 Non-specific ST-T changes). Abnormal ECG contained >200 cardiac diagnoses other than the ones introduced. Determined classes were STEMI and Non-STEMI. Algorithm was 1-D Convolutional Neural Networks. Gender and age were included in the study. Training/Testing showed 89% ECG (4,994 STEMI – 5,014 Not-STEMI) and 11% ECG (555 STEMI – 555 Non-STEMI), respectively. The last dense layer outputs the probability of each record associated to each class. The label of each record was executed; statistical values were analyzed through mean aggregation. The promising results are detected in these experiments such as accuracy of 95.8%; Sensitivity with 95.9%; Specificity of 95.7%.
Conclusion: AI capabilities may assist to faster and more efficient STEMI triage and management, which may verify particularly advantageous in more impoverished regions of the world that lack cardiologists to give urgent ECG interpretation.
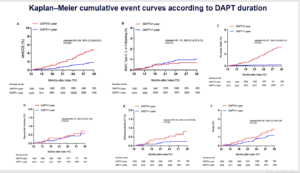
Benefits and Risks of DAPT Continuation Beyond 12 Months After PCI in Patients with High Thrombotic Risk Features as Proposed by 2018 ESC/EACTS Myocardial Revascularization Guideline
Wang HY, presented a study at TCT Connect 2020 virtual conference which analysed the benefits and risks of DAPT with aspirin and clopidogrel beyond 1 year versus ≤ 1-year DAPT on long-term clinical outcomes following PCI with DES with a prospective, real-world registry between ESC/EACTS guideline-endorsed HTR patients that are event-free at 12 months’ follow-up. A total of 10,724 consecutive patients who underwent PCI for CAD with DES implantation were potentially enlisted from January 2013 to December 2013. In brief, 5149 patients with HTR features were selected as per ESC/EACTS guideline-endorsed HTR criteria. 26 patients were excluded who were not followed up for 12 months after the index procedure and 545 patients who had experienced an adverse event [death, MI, stent thrombosis, repeat revascularization, stroke, or Bleeding Academic Research Consortium (BARC) major bleeding] at 12-month follow-up. The primary efficacy endpoint was major adverse cardiac and cerebrovascular events (MACCE) (a composite of all-cause death, MI, or stroke). The primary safety endpoint was clinically relevant bleeding defined as BARC type 2, 3, or 5 bleeding. Secondary endpoints included the individual components of the primary efficacy endpoint, cardiac death, target vessel MI, definite/probable stent thrombosis (ST), and BARC type 3 or 5 bleeding. Patients with DAPT > 1-year exhibited substantial reduction in all-cause death, cardiac death, MI compared to patients receiving DAPT ≤ 1-year. According to DAPT duration, in Kaplan–Meier cumulative event curves; patients with DAPT > 1-year showed significant reduction in MACCE, all-cause death, definite ST and stroke as compared to with DAPT ≤ 1-year.
Conclusion: In this real-world cohort including patients with ESC/EACTS guideline-endorsed HTR features who were event free for 1 year following coronary stenting, the continuation of DAPT beyond 1 year with clopidogrel and aspirin demonstrated a significant reduction in atherothrombotic events, featuring cardiac death, without apparent enhancement in clinically relevant bleeding risk when compared with ≤ 1-year DAPT. The findings may be informative for practicing cardiologist when contemplating extension of DAPT beyond 1 year following PCI in patients with ESC/EACTS guideline-endorsed HTR for protection from atherothrombotic ischemic events.
Contribution of ESC DAPT Guideline-Endorsed High Thrombotic Risk Features to Long-Term Clinical Outcomes Among Patients with and Without High Bleeding Risk After PCI
It remains unclear that whether the underlying risk of high bleeding risk (HBR) impacts the association of high thrombotic risk (HTR) attributes with adverse events following drug-eluting stent implantation. Wang HY, presented a study at TCT Connect 2020 virtual conference which assessed (1) the prognostic impact of ESC guideline-endorsed HTR features on long-term clinical outcomes and (2) whether the outcomes of HTR versus non-HTR features differ by HBR status. 10,167 consecutive patients were prospectively enlisted in Fuwai PCI Registry who underwent percutaneous coronary intervention among January 2013 and December 2013. Patients who are at HTR were categorised as: diffuse multivessel disease in diabetic patients, chronic kidney disease, at least three stents implanted, at least three stents lesions treated, bifurcation with two stents implanted, total stent length > 60 mm, or therapy of chronic total occlusion. The description of HBR was based on the Academic Research Consortium for HBR standard. The primary ischemic outcome was major adverse cardiac event (MACE), a composite of cardiac death, myocardial infarction, target vessel revascularization and stent thrombosis. The primary bleeding outcome was clinically relevant bleeding, described as per Bleeding Academic Research Consortium (BARC) type 2, 3 or 5 bleeding. 4430 patients (43.6%) with experienced HTR showed substantial higher risk of MACE ([HR] adjust: 1.56, 95% [CI]: 1.34-1.82; p<0.001) and device-oriented composite endpoint (composite of cardiac death, target-vessel MI, and target lesion revascularization) (HRadjust: 1.52 [1.27-1.83]; p<0.001) than those having non-HTR with a 2.4-year median follow-up. The risk of clinically relevant bleeding did not vary among groups (HRadjust: 0.85 [0.66-1.08]; p=0.174). HBR and non-HBR groups showed similar correlations between HTR and adverse events, without evidence of interaction (all pinteraction>0.05); but adverse event rates were greatest between patients with both HTR and HBR.
Conclusion: ESC guideline-endorsed HTR was correlated with substantially elevated risk of MACE without any substantial differences in clinically relevant bleeding. The presence of HBR does not appear as a modifier of cardiovascular risk for patients at HTR, recommending more vigorous and longer antiplatelet treatment may be advantageous for this patient population.


