COBRA-REDUCE: A Randomized Trial of a Thromboresistant Polyzene F-coated Stent with 14 Days DAPT in High-Bleeding Risk Patients
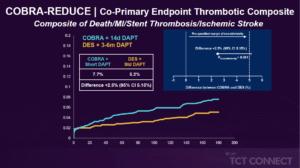
Byrne R, presented findings of the COBRA-REDUCE trial at the TCT Connect 2020 virtual conference. In patients receiving oral anticoagulation (OAC) undergoing coronary stenting, a coronary stent with thromboresistant and pro-healing properties such as the polymer polyzene F-coated (COBRA PzF) stent could safely allow for a very short period of triple therapy. The COBRA-REDUCE trial is a prospective, multinational, multi-centre, open-label, parallel group, and assessor-blinded, randomized trial. Patients at high risk of bleeding due to OAC requirement (vitamin K antagonist or non-vitamin K antagonist) were randomised to undergo COBRA-PzF stent therapy at the time of PCI, followed by a very short term (14 days) of dual antiplatelet therapy (DAPT) (Group A) or a new generation drug-eluting stent (DES) approved by the Food and Drug Administration (FDA) followed by guideline-recommended DAPT duration (3 or 6 months) (Group B). There were two co-primary endpoints: the bleeding co-primary endpoint is bleeding academic research consortium [BARC] ≥2 bleeding beyond 14 days or after hospital discharge; the thrombo-embolic co-primary endpoint was the composite of all cause death, myocardial infarction, definite or probable stent thrombosis or ischaemic stroke [non-inferiority hypothesis]). A total of 996 patients at high risk of bleeding due to OAC (vitamin K antagonist or non-vitamin K antagonist) criteria were randomised at 62 sites in the United States and Europe at the time of PCI; 495 patients were assigned to Group A, 501 to Group B. Between the treatment classes, baseline characteristics were well balanced. The mean age was 74.4 years, 27.0% were female, 36.2% had a diagnosis of diabetes mellitus, 12.1% had a history of previous stroke, 6.6% had known extreme renal insufficiency; 29.3% of patients presented with acute coronary syndrome. The endpoint BARC 2-5 bleeding after 14 days was lower in the group A (7.5%) vs. group B (8.9%). The co-primary endpoint composite of death/MI/stent thrombosis/ischemic stroke was also lower in-group A (5.2%) compared to group B (7.7%) [Difference +2.5% (95% CI 5.15%)]. The superiority analyses demonstrated lower incidence of BARC (2-5) and BARC (1-5) in-group B.
Conclusion: In patients undergoing PCI for acute or chronic coronary syndromes receiving oral anticoagulation, stenting with Cobra PzF and 14-day DAPT with or without decreased OAC severity did not minimise bleeding and did not meet non-inferiority requirements with respect to thrombotic events compared to regular DES and 3-6 months with DAPT.
HOST-REDUCE-POLYTECH-ACS: A Randomized Trial of Durable Polymer versus Bioabsorbable Polymer DES in Patients with Acute Coronary Syndromes
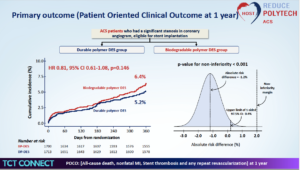
Kim HS, presented findings of the HOST-REDUCE-POLYTECH-ACS trial at the TCT Connect 2020 virtual conference. The objective of the study was to investigate the efficacy and safety of a biodegradable polymer DES versus durable polymer DES in patients with ACS undergoing PCI. 3429 acute coronary syndrome (ACS) patients were screened, out of which 16 were excluded due to randomization error. 1,713 patients were enrolled in the durable polymer DES group and 1,700 patients were enrolled in the biodegradable polymer DES group. Eligible patients with a culprit lesion in the native coronary artery or a graft vessel eligible for stent implantation with severe stenosis were randomised to robust polymer and absorbable polymer DESs in a 1:1 fashion. The working hypothesis of this study is that in reducing patient-oriented composite outcome (POCO), durable polymer DESs are not inferior to absorbable polymer DESs, described as a composite of all-cause death, nonfatal myocardial infarction, and any repeat revascularization at 12 months. The key secondary endpoint was device-oriented composite endpoint, a composite of cardiac death, target vessel myocardial infarction, or target lesion revascularization. The rate of POCO was 5.2% in the durable polymer DES compared to 6.4% in the biodegradable polymer DES group (HR 0.81, 95% CI 0.61-1.08, p=0.146). The rate of DOCO was marginally higher in the biodegradable polymer group (2.6% vs. 3.9% HR 0.67, 95% CI 0.46-0.98, p=0.038). The rate of secondary outcome (Device Oriented Clinical Outcome at 1 year) was 3.9% in the biodegradable polymer DES compared to 2.6% in the durable polymer DES group (HR 0.67, 95% CI 0.46-0.98, p=0.038). The rate of target lesion revascularization was 1.8 % in the biodegradable polymer DES compared to 1.0 % in the durable polymer DES group (HR 0.54, 95% CI 0.29-0.99, p=0.049).
Conclusion: In terms of 1-year POCO (patient driven composite outcomes), durable Polymer DES was non-inferior to Biodegradable Polymer DES. A sign of higher clinical events was observed in the Biodegradable than durable polymer DES with regard to DOCO (device driven composite outcomes).
IREMMI: Outcomes of MitraClip in Patients with Acute Mitral Regurgitation in AMI with and Without Cardiogenic Shock
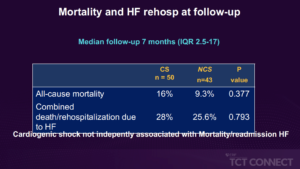
Loureiro ER, presented findings of the IREMMI trial at the TCT Connect 2020 virtual conference. The study was designed to report a multinational experience with MitraClip implantation in patients with acute MR following an AMI and cardiogenic shock (CS) at the time of the procedure, compared to those without shock. IREMMI was a prospective registry of all consecutive AMI acute MR patients treated with MitraClip in 18 centres in 8 European, Israeli and North American countries. 93 patients (51.6 % female, mean age 70.3±10.2 years) were included in the study between January 2016 and February 2020. Before the MitraClip procedure, 50 patients (53.8 %) had CS. CS patients appeared to be younger (68.5±10 vs. 72.5±10 years, p=0.061) relative to those without CS, with a lower left ventricular ejection fraction (34±12 % vs. 38±11 %, p=0.079). Similarly, the surgical risk was higher (Euroscore II 21.6±18% vs. 11.1± 8.4%, p=0.001) and the period from MI to clip seems to be shorter (24±22 vs. 33± 2%, p=0.001). CS patients had higher frequency of vasopressors (83% vs. 4%, p<0.001), IABP/Impella (66% vs. 6%, p<0.001) and VA ECMO (12% vs. 0%, p=0.028). In 86 % of CS and 79 % of non-CS, preprocedural MR was 4 + (p=0.377). There was no difference in procedural performance between groups of 90 % vs. 93 % (p=0.793), but procedures in CS were longer (143±113 vs. 83±44 minutes, p=0.003). Major complications after procedure did not vary between groups (4% vs. 7%, p=0.659). There were no major changes in mortality during the 30-day follow-up (10 % vs. 2.3 %, p=0.212). At 3-month follow-up MR grade ≤2+ was documented in 83.4% in CS and 90.6% in non-CS patients (p=0.348), sPAP was comparable between groups (39.6±13 vs. 44±19 mmHg, p=0.441) and NYHA functional class was I-II in 79.5% in CS and 86.5% in non-CS (p=0.608). The total mortality did not vary between groups after a median follow-up of 7 months (range 0-81 months) (16 % CS vs. 9.3 % non-CS, p=0.377), and the combined incident mortality/rehospitalization due to heart failure was comparable (28 % CS vs. 25.6 % non-CS, p=0.793). CS was not individually correlated with the aggregate end point (HR 1.1, CI 95 % 0.3-4.6) in a Cox-regression analysis adjusted for age, Euroscore II and procedural performance.
Conclusion: In patients who experience acute MR after AMI, cardiogenic shock does not tend to affect the effects of MitraClip. In this case, CS development does not preclude percutaneous mitral valve repair.
OPTIMIZE: A Randomized Trial of a Novel, Ultra-low Profile Fixed-wire DES
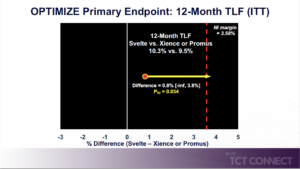
Kereiakes D, presented findings of the OPTIMIZE trial at the TCT Connect 2020 virtual conference. OPTIMIZE was a prospective, single-blind, randomized, international, multi-center trial comparing the new stent to existing drug-eluting stents (Xience® or Promus® EES) in 1,639 subjects with ischemic heart disease with 3 or less de novo stenotic lesions (34 mm or less in length) in 2 or less native coronary arteries with RVD 2.25 mm – 4.00 mm amenable to PCI. The primary endpoint, target lesion failure (TLF), was powered for noninferiority at 12 months. 1,639 subjects (72 % male, 30 % diabetic, 57 % acute coronary syndrome, mean age 65.4 years) with 1,988 lesions (74 % Type B2 / C, 36 % moderate-severely calcified, 20 % vessel angulation > 45 °, mean stenosis 64 %, lesion length 14.6 mm, RVD 2.78 mm) were 1:1 randomised at 74 US study sites (57 %) from January 2018 to June 2019. The TLF rate was 10.3% in the Svelte group and 9.5 % in the Xience / Promus group, at 12 months (difference = 0.8 % [-inf, 3.8 %] PNI = 0.034, which was above the recommended 0.025 threshold for non-inferiority). For the secondary outcome, no variations were found between the two groups, 12-month TLF components, clinically suggested target lesion revascularization (TLR): 1.52 % vs. 1.93 %, p=0.57; cardiac death: 0.25 % vs. 0.26 %, p=1.00; TVMI: 9.3 %, p=0.57; cardiac death: 0.25% vs. 0.26%, p=1.00; TVMI: 9.31% vs. 8.22%, p=0.48. TLF (9.9%) was driven by TVMI (8.8%); 90% of TVMI was peri-procedural and 25% of subjects with troponin assays accounted for 80% of TVMIs. OPTIMIZE study non-inferiority is met applying the SCAI definition of MI or a relative NI margin using the protocol definition of MI.
Conclusion: No differences between Xience/Promus and Svelte DES were observed for any primary or secondary endpoints (including the very low rate of TLR and stent thrombosis) in this ‘more comers’ study population. Based on the prespecified study statistical analysis plan, Svelte DES did not meet the threshold for non-inferiority using the prespecified absolute non-inferiority margin.
PARTNER 2: Five-Year Follow-Up from the Aortic Valve-in-Valve Registries
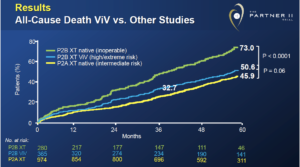
Hahn R, presented findings of the PARTNER 2 trial at the TCT Connect 2020 virtual conference. The PARTNER 2 Valve-in-Valve (ViV) registries (initial = 96 patients and ongoing access = 269 patients) enrolled patients at high risk of re-operation with failed surgical bioprostheses who were appropriate for 23 or 26 mm SAPIEN XT valves. In 95.9 % of patients, five-year clinical and echocardiographic follow-up was complete in patients with established visit accountability. Mean age was 78.9 ± 10.2 years in 365 ViV patients, mean STS score was 9.1 ± 4.7, and NYHA functional class was III or IV in 90.4 % patients. The Kaplan- Meier rates of all-cause mortality and any stroke were 50.6% and 10.5%, respectively, at 5 years. Structural valve deterioration (SVD)-related hemodynamic valve deterioration or bioprosthetic valve failure at 5 years was 6.6 % using the new VARC-3 definitions. In 6.3 % (n=14), aortic valve re-replacement was performed, the majority of which was due to restenosis (n=6) and combined aortic insufficiency/paravalvular regurgitation (n=3). Mean gradient, Doppler velocity index, paravalvular regurgitation, and quality of life assessed in survivors by KCCQ scores remained constant from 30 days to 5 years post-procedure. These findings are comparable to the 5-year TAVR outcomes in patients with PARTNER 2A intermediate-risk native SAPIEN XT (mortality 46.0%, any stroke 15.3%, SVD-related valve deterioration or failure 9.5%).
Conclusion: Valve-in-Valve TAVR mortality at 5 years is equivalent to that seen in intermediate-risk native TAVR patients. Early change in functional status and quality of life has been consistent in survivors for 5 years. The HVD and BVF rates in intermediate-risk patients were consistent with those reported for native SAPIEN XT valves.
TRANSIT: Treatment of Failed TAVR with TAVR
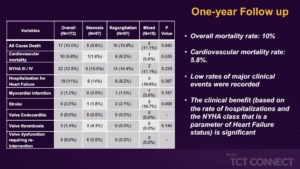
Testa L, presented findings of the TRANSIT trial at the TCT Connect 2020 virtual conference. The study was conducted to evaluate the outcomes of patients with a degenerated TAV treated by means of a second TAVR. The TRANSIT (Transcatheter aortic valve replacement for failed transcatheter aortic valves) project is an investigator-initiated registry that began collecting data in January 2020. Initially, more than 60 centres worldwide were contacted to participate in the final group of 28 centres: 22 in Europe, 4 in North America, 1 in South America and 1 in the Middle East. Among the total number of approximately 40,000 procedures carried out since 2008 in enrolling centers, 172 cases have been collected: patients were analysed according to the mode of failure of the first bioprosthetic valve (57 – stenosis; 97 – regurgitation; 18 – mixed). Overall, the presentation rate of NYHA class III / IV was 73.5 %. As a result of residual gradient (14 %) or regurgitation (7 %), the VARC-2 device success rate was 79 %. The overall mortality rate was 2.9 % at 1-month, while new hospitalization rates and NYHA class III / IV rates were 3.6 % and 7 %, respectively, significant difference across the groups. Of note, there were 3 reported cases of valve thrombosis. The overall mortality rate was 10 % at 1 year, while new hospitalisation rates and NYHA class III/IV were 7.6% and 5.8%, respectively, without significant difference across the groups. No further cases of valve thrombosis were recorded.
Conclusion: By means of a second TAVR, patients with degenerated TAV can be treated safely and successfully. For the adoption of TAVR technology in a lower risk and younger population, this finding is of critical importance.
SCOPE I: One-year Results from a Randomized Trial of Self-expanding versus Balloon-expandable TAVR
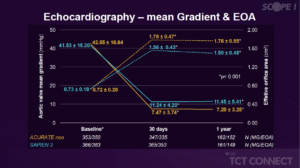
Walther T, presented findings of the SCOPE I trial at the TCT Connect 2020 virtual conference. In patients undergoing transcatheter aortic valve replacement (TAVR) for symptomatic severe aortic stenosis, SCOPE I is the first randomised clinical trial evaluating the safety and efficacy of self-expanding ACURATE neo versus a balloon-expandable SAPIEN 3 device. Compared to the SAPIEN 3 device, TAVR with ACURATE neo did not comply with non-inferiority in terms of primary composite safety and clinical efficacy endpoint at 30 days due to higher rates of moderate or severe prosthetic aortic regurgitation and acute kidney injury. Participants were randomly allocated (1:1) to treatment with the ACURATE neo or the SAPIEN 3 device on the basis of a randomly permuted block regimen. Assessors masked to treatment allocation independently adjudicated clinical events. Clinical outcomes at 1 year were compared between the two treatment arms by means of Cox proportional hazard models. Valve-hemodynamic performance as well as functional outcomes was assessed at 1 year. Analyses of clinical endpoints were performed according to the intention-to-treat principle; echocardiographic outcomes are site-reported for the valve-implant cohort. Of 355 patients, 40 (11.1 %) out of 360 in the ACURATE neo group and 30 (8.5 %) out of 355 in the SAPIEN 3 group died over one year ([HR] 1.32, 95 % CI 0.82 to 2.12). 17 (4.7%) of 358 in the ACURATE neo and 15 (4.2%) of 356 patients in the SAPIEN 3 arm suffered a stroke (HR 1.14, 95% CI 0.57 to 2.29). The occurrence of moderate or severe paravalvular regurgitation in 44 (6.1%) of the patients after TAVR at 30 days was not associated with all-cause mortality at 1 year. Mean transprosthetic gradient was lower (7.9 vs. 12.5, p < 0.001) and mean effective orifice area higher (1.74 vs. 1.57, p < 0.001) with ACURATE neo at 1 year.
Conclusion: In patients undergoing transfemoral TAVR SCOPE I, one-year clinical and functional results did not vary significantly between ACURATE neo and the SAPIEN 3 platform. The PVR rate remained higher in terms of valve efficiency, but the hemodynamic profile of patients treated with ACURATE neo was better with lower transprosthetic gradients and higher effective orifice at 1 year.
Impact of DAPT Cessation in High Bleeding-Risk Patients Undergoing PCI: Insights from the PARIS Registry
Dual antiplatelet therapy (DAPT) treatment in patients at high risk of bleeding (HBR) is difficult. Davide Cao, presented a study at TCT Connect 2020 virtual conference which analysed the incidence, patterns, and clinical effect of DAPT cessation in patients undergoing percutaneous coronary intervention (PCI) as per the presence of HBR. To distinguish 5,018 patients from the PARIS registry (Patterns of Non-Adherence to Anti-Platelet Regimens in Stented Patients Registry) into HBR (27.8%) and non-HBR groups (72.2%), the Academic Research Consortium criteria for HBR were used. Modes of DAPT cessation arising all over the 2-year follow-up were categorised as physician-suggested discontinuation, temporary interruption (<14 days), and disturbance because of bleeding or noncompliance. HBR patients were older, were more commonly women, and showed more cardiovascular risk factors and comorbidities as compared to non- HBR patients. Moreover, HBR patients showed higher occurrence of major adverse cardiac events (MACE) (composite of cardiac death, myocardial infarction, or definite/ probable stent thrombosis) and Bleeding Academic Research Consortium type 3 or 5 bleeding at 2 years than non-HBR (12.4% vs. 4.3%; p < 0.001 and 8.9% vs. 2.3%; p < 0.001, respectively). DAPT cessation was also more frequent in HBR patients because of greater rates of discontinuation, interference, and disturbance. Compared to uninterrupted DAPT, DAPT disruption was correlated with an enhanced risk of MACE, while discontinuation and interruption were not. The effect of DAPT cessation modes on adverse events was similar among HBR and non-HBR patients.
Conclusion: In HBR patients, DAPT cessation happens more frequently as compared to non-HBR patients, however its effect on cardiovascular outcomes is compatible nevertheless of HBR status.
Comparison of a Novel Biodegradable Polymer Sirolimus-Eluting Stent with a Durable Polymer Everolimus-Eluting Stent: A Systematic Review and Meta-Analysis
Since local vascular inflammatory reactions and stent thrombosis have been linked with durable polymers, biodegradable polymers are desirable because the drug is eluted and the bare-metal stent is left in the vessel. However, individual tests have shown indeterminate findings with respect to which stent is better. Khalid Y, presented a study at TCT Connect 2020 virtual conference which explored whether the newest biodegradable polymer ultrathin sirolimus-eluting stent (SES) is better to the durable polymer everolimus-eluting stent (EES) with regard to long-term individual clinical results at 1-year follow-up. From 7 trials, an accumulated data meta-analysis of the final results comparing SES to EES was executed. A random-effects model computed relative risk and 95% confidence intervals. Total 8,721 patients were enrolled which obtained either SES (n = 4,596) or EES (n = 4,125). No statistically substantial difference was observed in cardiovascular incidents and mortality (p = 0.624), event of any myocardial infarction (p = 0.125), target vessel myocardial infarction (p = 0.112), or target lesion revascularization (p = 0.145) between the two groups. This was true for stent thrombosis as well, however there was a very low altogether rate of stent thrombosis itself (<2% for both SES and EES).
Conclusion: Coronary implantation of SES exhibited no difference in clinical outcomes as compared to EES in this large dataset. To continue exploring the comparison of both of these stents, future studies are required.
Computational Modeling to Assess Efficacy of Drug-Coated Balloon Therapy
In the treatment of restenosis, drug-coated balloons have demonstrated positive results. However, evidence to justify its use for de novo coronary atherosclerotic lesions is still lacking. While there is available scientific and clinical evidence, the lack of “class effect” on the uptake mechanism and therapeutic effectiveness is not well understood. Toh HW, presented a study at TCT Connect 2020 virtual conference which assessed the effectiveness of drug coated balloons with a combined in silico and experimental approach. Three different bench-top experiments were executed with balloons coated with proprietary formulation including sirolimus and a hydrophilic excipient. In a computational model, these experimental results were used to solve a finite-element problem outlining a diffusion-advection-reaction problem to forecast the uptake of sirolimus. Tracking loss was estimated to be about 23.17% and adhesion efficacy was estimated to be approximately 19.55%. 17.02% of the coated sirolimus was transferred into the vessel segments after 10 min via computational modeling. Furthermore, the computational model also reflected a logarithmic increase in drug uptake in correlation to enhanced balloon expansion time, suggesting to the possibility of an ideal therapeutic window for the method.
Conclusion: The combined perspective proposed in the study may be able to precisely forecast the in vivo performance of sirolimus-coated balloons based on the consequences. This procedure could be a more robust means of estimating performance of drug-coated balloons and prospectively estimate their effectiveness as a therapeutic alternative.

Optimal Duration of Dual Antiplatelet Therapy After Percutaneous Coronary Intervention in Patients with Acute Coronary Syndrome: Insights from a Network Meta-Analysis of Randomized Trials
In patients with stable coronary artery disease, the minimal duration of dual antiplatelet therapy (DAPT) suggested using guidelines has been decreased to 6 months with newer generation drug-eluting stents. It is unclear whether the shorter duration of DAPT is effective in patients with acute coronary syndrome (ACS). Kuno T, presented a study at TCT Connect 2020 virtual conference which explored the optimum DAPT duration (≤ 3 months vs. 6 months vs. 12 months vs. >12 months) between patients with ACS undergoing PCI. PubMed and Embase were scanned for randomized controlled trials of DAPT duration in patients with ACS. The ischemic outcomes were all-cause death, myocardial infarction, and stent thrombosis. The safety outcome was major and/ or clinically pertinent bleeding. 14 eligible trials were recognised in this search; a total of 31,837 patients were enlisted comparing different DAPT duration in patients with ACS. Short-term DAPT (≤3 months or 6 months) did not show an increase in ischemic outcomes as compared to long-term DAPT (12 months and >12 months). In bleeding outcomes, DAPT duration ≤ 3 months was correlated with substantial reduction in bleeding as compared to DAPT durations of 6 months, 12 months, or > 12 months ([HR]: 0.60; 95% [CI]: 0.37 to 0.98; HR: 0.68; 95% CI: 0.54 to 0.85; and HR: 0.43; 95% CI: 0.34 to 0.54, respectively). These findings were alike when restricted to second-generation drug-eluting stents.
Conclusion: In patients with ACS undergoing PCI, the data from this meta-analysis of randomized trials assist short-term DAPT (≤3 months and 6 months). Even in patients presenting with ACS, guidelines should contemplate short-term DAPT, particularly in this period of newer generation drug-eluting stents.
In-hospital Outcomes of CTO PCI in Octogenarians and Nonagenarians: Insights from the PROGRESS-CTO Registry
There has been minimal study on the outcomes of chronic complete occlusion (CTO) percutaneous coronary intervention (PCI) in octogenarians and nonagenarians. Vemmou E, presented a study at TCT Connect 2020 virtual conference which compared in-hospital outcomes of CTO PCI among patients ≥ 80 years and < 80-years-old. 6,233 CTO PCIs were executed in 6,050 patients among 2012 and 2020 at 33 U.S. and international centers. There were 415 octogenarians and nonagenarians in the study (7% of the total population). Octogenarians and nonagenarians were less likely to be men (73% vs. 83.2%, p < 0.0001) and showed more atrial fibrillation (27% vs. 12%, p < 0.0001) and prior coronary artery bypass graft surgery (43% vs. 29%, p < 0.0001) as compared to younger patients. They were more likely to have CTOs with moderate/severe calcification (71% vs. 46%, p < 0.0001), however they had similar mean J-CTO scores (2.5 ± 1.3 vs. 2.4 ± 1.3, p = 0.09). The most frequent crossing approach used was antegrade wire rise in both groups (84% in octogenarians/nonagenarians vs. 85% in younger patients, p = 0.64). Non-CTOs were managed in 32% of patients in the ≥ 80-year-old group as compared to 25% in the < 80-year-old group (p = 0.018) at the same time. Octogenarians and nonagenarians showed more balloon uncrossable (17% vs. 10%, p = 0.0006) and balloon undilatable lesions (15% vs. 9%, p = 0.006). The octogenarians/ nonagenarians showed lower technical and procedural success (82.2% vs. 86.3%, p = 0.02, 80.3% vs. 84.8%, p = 0.016, respectively) and greater occurrence of in-hospital major adverse cardiovascular events (MACE) (3.4% vs. 1.8%, p = 0.02). Most in-hospital MACE occurrences were cardiac tamponade needing pericardiocentesis.
Conclusion: In octogenarians and nonagenarians, CTO-PCI is viable, while success rates are lower, and the risk of complications is greater as compared to younger patients.


