EMPEROR-Reduced: Empagliflozin in Heart Failure With a Reduced Ejection Fraction, With and Without Diabetes
Packer M, at the ESC Congress, 2020: A digital expereince, presented the full results from the EMPEROR-Reduced Phase III trial in adults with heart failure with reduced ejection fraction, with and without diabetes. EMPEROR-Reduced trial demonstrated that empagliflozin was associated with a significant 25 % relative risk reduction in the primary endpoint of time to cardiovascular death or hospitalization due to heart failure. The trial evaluated the effect of adding empagliflozin (10 mg) versus placebo to standard of care. 3730 patients were enrolled in this trial and 1863 patients were randomized to Empagliflozin and 1869 were randomized to placebo. Consistent results were observed from the primary endpoint in the subgroups with and without type 2 diabetes. The trial further demonstrated that empagliflozin reduced the relative risk of first and recurrent hospitalization for heart failure by 30 %. Furthermore, the rate of decline in eGFR, a measure of kidney function decline, was slower with empagliflozin than with placebo.
The absolute risk reduction assessed in an exploratory analysis, observed that the primary endpoint of EMPEROR-Reduced corresponded to a number needed to treat of 19 patients over 16 months to prevent one cardiovascular death or hospitalization for heart failure. Further, an additional exploratory analysis proved that empagliflozin reduced the relative risk of a composite kidney endpoint , comprising of end stage kidney disease and a significant loss of kidney function, by 50 %. In EMPEROR-Reduced, the efficacy results were achieved with a simple dosing regimen, with once daily dosing with no need for titration. The safety profile was similar to the well-established safety profile of empagliflozin. There were no clinically meaningful differences in adverse events including hypovolemia (decreased blood volume), hypotension (low blood pressure), volume depletion (loss of fluids), renal insufficiency (poor kidney function), hyperkalemia (high potassium levels) or hypoglycemic events (low blood sugar) compared with placebo.
Conclusion: EMPEROR-Reduced trial demonstrated that patients with chronic heart failure and a reduced ejection fraction, achieved all three endpoints prespecified as key outcomes by heirarchial testing, each with p < 0.001. Combined together, the consistent results of DAPA-HF and EMPEROR-Reduced ought to be appropriate to ascertain SGLT2 inhibitors as the standard of care for heart failure patients with reduced ejection fraction.
Expanding Understanding For Patients With Diabetes and Cardiovascular Disease – Results From The VERTIS CV Trial
Cannon et al., presented the results of VERTIS CV Trial at ESC congress 2020. VERTIS CV is a multicenter, randomized, double-blind, placebo-controlled, event-driven trial to evaluate the metabolic and cardiovascular effects of Ertugliflozin, including the cardiovascular meta-analysis, and implications for patients with diabetes and established cardiovascular disease. All the patients were randomized into 3 groups: a) Placebo b) Ertugliflozin 5mg c) Ertugliflozin 15mg. The study results depicted CV Outcomes in MACE wherein MACE efficacy across class was generally modest, EMPA-REG OUTCOME was significant on MACE due to effect on CV death and no effect on MI or stroke. CANVAS was significant on MACE due to contribution from MI, CV death and stroke with this, DECLARE and VERTIS CV only found trend on MACE. With respect to CV Death, Only EMPA-REG OUTCOME found significant reduction, driving heterogeneity in the beneficial effect for the class. With HHF, consistent effects across the class were substantial. Benefits were found to be independent of baseline ASCVD, prior HF and across spectrum of baseline eGFR. In renal outcomes, although other CV outcomes trials showed renal outcomes benefit, VERTIS CV showed a trend in the initial renal analysis. It demonstrated the following: Effect on kidney-related outcomes generally consistent with other SGLT2 inhibitor trials, effects on acute and chronic eGFR consistent with other SGLT2 inhibitors and renal AEs in line with SGLT2 inhibitor class.
Conclusion: The author concluded the study with VERTIS CV achieving its primary endpoint of non-inferiority for MACE compared with placebo in patients with T2DM and established ASCVD, demonstrating the CV safety of ertugliflozin. VERTIS CV provided evidence of CV safety of SGLT2 inhibitors for the treatment of patients with T2DM and added to the evidence of benefit on HHF consistent across the class. Ertugliflozin was well tolerated, with a safety profile consistent with previous studies of ertugliflozin. The Meta-analyses supported the contemporary society recommendations to prioritize the use of SGLT2 inhibitors, independent of glucose control consideration, in patients with T2DM with or at high risk of CV and renal complications.

Reversing Cardiac Remodelling with Heart Failure treatment
Januzzi J, presented a session on reversing cardiac remodelling with heart failure treatment at the ESC Congress, 2020: A digital experience. Cardiac remodelling is a pivotal process occuring in heart failure and is associated with progression of disease. Remodelling includes molecular, cellular, and interstitial events that contribute to clinically relevant changes in cardiac structure, function, or both. These changes affect function and may occur in cardiac chamber dimensions, wall thickness, volume, mass and ejection fraction. Although initially adaptive, sustained remodelling leads to progressive, irreversible dysfunction. Cardiac remodelling occurs in response to patho-physiological stimuli. If unchecked, cardiac remodelling leads to symptomatic heart failure. Cardiac remodelling in HFrEF is associated with an increased risk of CV events in patients with HF. In contrast, reverse remodelling in HFrEF, judged by reduced LV size and improved function is associated with improved symptoms and prognosis, lower risk of hospitalizations and death and better quality of life. The variables that predict reverse cardiac remodelling are therapies such as guideline directed medical therapy and Cardiac resynchronization therapy. Heart failure therapies which leads to reverse modelling also foster significant improvement in prognosis. Biomarkers such as lower NT-proBNP, hs-cTn, sST2 also predict presence and magnitude of reverse cardiac remodelling. Concentration of NT-proBNP reduction is strongly associated with greater decrease of cardiac reverse remodelling manifesting as improvement in ejection fraction and reduction in left ventricular volume(LVV). In PROVE-HF study, patients with larger and faster reduction in NT-proBNP and LVESVi by 6 months had lowest rate of subsequent death or HF hospitalization by 12 months.
Conclusion: Progressive, “forward” remodelling of the heart is a pivotal aspect of HFrEF progression and linked to risk for events. Reverse remodelling is associated with lower event rates. Therapies with favourable effects in HFrEF also tend to variably foster reverse remodelling. Among available therapies that have the most substantial reverse remodelling effects are CRT, beta blockers and Sacubitril/valsartan.

Empagliflozin impairs smooth muscle cell proliferation, accelerates endothelial regeneration, and prevents neointima formation by altered expression of the vasoactive peptide apelin
An inhibitor of the sodium glucose co-transporter 2 (SGLT2), Empagliflozin an anti-diabetic agent exerts further advantageous impacts on heart failure outcomes in patients with type 2 diabetes mellitus at high cardiovascular risk. But, the impact of Empagliflozin on vascular cell function and vascular remodeling activities remains largely elusive. Dutzmann J, et al., presented a study at ESC Congress 2020: A Digital experience which analysed the effect of Empagliflozin on vascular smooth muscle (SMC) and endothelial cells (EC).
Immunocytochemistry and immunoblotting exhibited SGLT2 to be considered in human diabetic and non-diabetic SMC and EC as well as in murine femoral arteries. In vitro, Empagliflozin substantially decreased serum-induced proliferation and movement of human diabetic and nondiabetic SMCs in a dose-dependent manner without any toxic or apoptotic impacts. In contrast, Empagliflozin substantially elevated the cell count and migrational capacity of human diabetic ECs, however not of human non-diabetic ECs. In vivo, therapeutic application of Empagliflozin (225 mg/kg medicated diet) showed substantial reduction in number of Ki-67+ proliferating neointimal cells in response to femoral artery wire-injury in C57BL/6J mice. In addition, Empagliflozin inhibited following neointima formation (luminal stenosis 91.2% vs. 80.6 % at 21 days; p<0.05). In a streptozocin-induced diabetic model of apolipoprotein E-/- mice, comparable effects of Empagliflozin were seen. Conclusive to the in vitro-outcomes, re-endothelialization was not substantially impacted in C57BL/6 mice (non-re-endothelialized distance 2.57 mm vs. 2.3 mm; p=0.07). The Empagliflozin treatment decisively regulated vasoactive peptide apelin in microarray analysis of human SMCs. Further pathway analysis showed apelin to inhibit SMC proliferation with de-phosphorylation of Akt and to increase EC proliferation with phosphorylation of p38 MAPK.
Conclusion: For the first time these data document the functional effect of Empagliflozin on vascular SMCs and ECs. Empagliflozin substantially decreases serum-induced proliferation and migration of SMCs in vitro and inhibited neointima formation in vivo, altough increasing EC proliferation in vitro and re-endothelialization in vivo following vascular injury. Hence, Empagliflozin carries promise to showed favorable impacts on vascular healing, and to inhibit neointima formation after vascular injury in diabetic and non-diabetic patients.
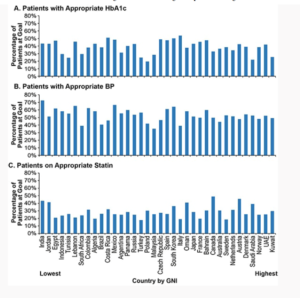
Effects of canagliflozin on cardiovascular death and hospitalization for heart failure by baseline estimated glomerular filtration rate: integrated analyses from the CANVAS Program and CREDENCE
People with type 2 diabetes mellitus (T2DM) exhibit higher risk for cardiovascular (CV) disease, including hospitalization for heart failure (HHF), a complication that is more frequent as renal function decrease. The sodium glucose co-transporter 2 (SGLT2) inhibitor Canagliflozin (CANA) decreased the risk of HHF in patients with T2DM and high CV risk or nephropathy in the CANVAS Program and CREDENCE trials, respectively. Mahaffey KW, et al., presented a study at ESC Congress 2020: A Digital experience which integrated analyses from the CANVAS Program and CREDENCE.
This post hoc investigation incorporated increased, pooled data from the CANVAS Program and the CREDENCE trial. The impacts of CANA were compared with placebo on CV death or HHF, HHF, and CV death were examined in subgroups described with baseline eGFR (<45, 45-60, and >60 mL/min/1.73 m²). Hazard ratios (HRs) and 95% confidence intervals (CIs) were analysed by Cox regression models, with subgroup by treatment interaction terms added to test for heterogeneity. Interaction p values were estimated by incorporating treatment group and baseline eGFR in the model.
A total of 14,543 participants were included with mean age of 65 y; 65% male; 75% white; mean eGFR 70.3 mL/min/1.73 m² from the CANVAS Program (n=10,142) and CREDENCE (n=4,401). 1919 (13.2%) patients showed baseline eGFR <45 mL/min/1.73 m² (mean, 36.7 mL/min/1.73 m²), 2972 (20.4%) patients showed eGFR 45-60 mL/min/1.73 m² (mean, 53.1 mL/min/1.73 m²), and 9649 (66.3%) patients showed eGFR >60 mL/min/1.73 m² (mean, 82.3 mL/min/1.73 m²). Rates of CV death or HHF, HHF, and CV death were enhanced as eGFR decreased (Figure). CANA exhibited substantial reduction in the risk of CV death or HHF and HHF as compared to placebo (PBO), with compatible impacts observed over subgroups.
Conclusion: An occurrence rate of CV death or HHF, HHF, and CV death were enhanced with lower baseline eGFR. CANA substantially decreased the risk of CV death or HHF, jointly and individually, with compatible advantages observed despite baseline eGFR in participants with T2DM and high CV risk or CKD in the CANVAS Program and the CREDENCE trial.
The effect of SGLT2 inhibitors on silent myocardial ischemia
Sodium-glucose co-transporter 2 (SGLT2) inhibitors decreased the development of heart failure (HF) in patients with type 2 diabetes (T2DM) in large cardiovascular (CV) outcome trials. Also in HFrEF patients, it decreased CV death and worsening HF occurrences. However, whether SGLT2 inhibitors work through glucose dependent procedure or it could have other impacts not linked to glucose on cardiovascular morbidity and mortality in diabetic patients, is not known. Also, its impact on silent ambulatory myocardial ischemia (SAMI) has not been outlined yet. Kadro W, et al., presented a study at ESC Congress 2020: A Digital experience which reported the impact of SGLT2 inhibitors on (SAMI). Treatment of silent myocardial ischemia show a prognostic impact and may enhance long term mortality of chronic ischemic heart disease (CIHD).
44 T2DM patients with proven stable coronary artery disease (CAD) and at least one episode of on silent ambulatory myocardial ischemia (SAMI) on ambulatory ECG monitoring were enrolled. All of them were taking optimal treatment for CIHD and type 2 DM. 22 patients were randomized to take Dapagliflozin 5mg qd and the other 22 patients received placebo. After 4 to 6 months of treatment, ambulatory monitoring was repeated. The two groups were compared with respect to baseline characteristics, number of episodes of ST-segment depression, HbA1c level, and baseline serum cholesterol levels. A blinded cardiologist read the Holters.
The Dapagliflozin group showed a substantial reduction in the number of episodes of ST-segment depression as compared to the placebo group. 8 of 22 patients (36%) completely resolved the ST-segment depression in the Dapagliflozin group versus 3 of 22 (13%) in the placebo group.
The Dapagliflozin group showed a greatly substantial reduction in (SAMI) (p<.001). The Dapagliflozin therapy was an independent predictor of (SAMI) resolution by logistic regression. Possible procedures for this advantageous impacts of SGLT2i includes: Reduction in oxygen supply-demand mismatch, enhancement in cardiac microvascular function, modification of cardiac energy metabolism, decrease in glucotoxicity, sympathetic nervous system activation and blood pressure.
Conclusion: Treatment with SGLT2 inhibitors showed reduction or resolution of SAMI recorded as episodes of ST-segment depression in ambulatory monitoring of the ECG in T2DM patients. A larger study is needed to confirm this theory and to identify the impact of SAMI decrease on long term mortality of CIHD in diabetics.
Chronic Heart Failure – Essential Update
Peter Seferovic presented his study focusing on drug treatment for chronic heart failure at the ESC Congress 2020: A Digital Experience. As per the 2016 ESC Guidelines, the treatment of heart failure starts with beta blockers and ACE inhibitors. Presenting one of the position statement from heart failure Association of the European Society of Cardiology, the author presented use of diuretics in heart failure. The pharmacologic treatments indicated in NYHA II HFrEF ACE-I is recommended in addition to beta blocker for symptomatic patients with HFrEF to reduce risk of HF hospitalization and death. In addition a beta blocker is also recommended in addition to ACE-I. The PARADIGM-HF primary results showed significant reduction in primary endpoints CV death or heart failure hospitalization with Enalapril. In addition the author also said that SGLT2 inhibitors effectively reduce the risk of HF hospitalization regardless of a history of HF. DAPA HF also depicted dapagliflozin reducing CV death or worsening of HF compared to placebo.
Prof Maria from Spain talked about current management of HF. The author initially depicted about normal status and advanced HF in Hemodynamic progression of HF. To recognize such patients the speaker presented a chart with I NEED HELP. The speaker also classified the cardiogenic shock as A T Risk, Beginning, Classic & Deteriorating. In order to define HF, the speaker mentioned about 2018 HF –ESC criteria for defining Advanced HF. The speaker mentioned about MCS-options to pump blood within the CV system, heart transplantation and LT-MCS. The Hub & Spoke model depicting different heart failure units between patients and medical therapy.
Conclusion: Collectively it can be concluded that drugs and devices both should be included in HF treatment. In advanced heart failure treatment, decision making with evaluation for heart transplantation and LVAD implantation must be done.
Management of patients with mid-range ejection fraction: gaps in Guidelines
Anker S, at the ESC Congress, 2020: A digital expereince, presented a session on management of patients with mid-range ejection fraction (EF): gaps in guidelines. The guidelines were developed early in 2016, the most interesting development was the mid-range EF, new group of HF patients that lie between HFrEF and HFpEF, when the patient has EF of 40-49% The actual treatment given to HFmrEF patients is like the treatment of HFrEF patients; mostly with diuretics (47-74%) and loop diuretics (77-90%), ACEi/ARB, beta blockers and MRAs. Only 1-7% patients undergo implantable cardioverter-defibrillators (ICD) and 1-11% patients undergo cardiac resynchronization therapy (CRT). Generally, HFmrEF patients are considered as HFpEF patients, but the guidelines do not make any statements beyond giving diuretics for symptoms and treat co-morbidities. From the 2019 HFA clinical practice update, in the treatment of HfmrEF:
- A beta-blocker may be considered for ambulatory patients with symptomatic HFmrEF in sinus rhythm in order to reduce the risk of all-cause and CV death
- Candesartan may be considered for ambulatory patents with symptomatic HFmrEF in order to reduce the risk of HF hospitalization and CV death
- Spironolactone may be considered for ambulatory patients with symptomatic HFmrEF without contraindications in order to reduce the risk of CV death and HF hospitalization
In the combined PARADIGM and PARAGON trial, there was no significance for women with LVEF >55% and for men with LVEF>33%. Below EF 50%, sacubitril/valsartan seems to work in men and women with HF (incl. HFmrEF).
Conclusion: Based on the findings of clinical trial and the need to reduce the adverse consequences of heart failure on public health, serious consideration should be given to increasing the LVEF threshold for the use of evidence-based treatments from its current value of 40% to value of 50%.

Hemodynamic and metabolic disorders in obese patients with resistant hypertension
Resistant hypertension (RH) is a significant origin of cardiovascular morbidity and mortality. Both true and pseudo-resistant arterial hypertension (AH) show a major issue not only in blood pressure (BP) control, however also in those possible adverse cardiovascular occurrences, the progression of which is correlated with failure to reach target BP levels.
Shalimova A, et al., presented a study at ESC Congress 2020: A digital experience which initiated the attributes of hemodynamic and metabolic parameters in obese patients with true and pseudo-resistant AH.
200 patients were included in the study with uncontrolled AH and obesity. Initially dual antihypertensive treatment was prescribed to patients. Those patients who did not achieve target BP levels after 3 months on dual therapy were further allocated a third antihypertensive drug. From the 98 patients who were allocated triple therapy, 48 patients did not achieve target BP (27 patients showed pseudo-resistant and 21 patients showed true resistant AH). These patients were further prescribed a fourth antihypertensive drug (spironolactone). The efficacy of the therapy was analysed for 6 months after the begin of antihypertensive therapy.
After 6 months of treatment, patients with RH showed greater body mass index (BMI) and greater BP levels than patients without this condition. Patients with RH also showed more proclaimed cardiovascular remodeling, greater levels of triglycerides, insulin, HbA1c, more pronounced insulin resistant (IR), which was affirmed with higher HOMA-IR, higher imbalance of adipokines, proinflamation activity and greater activity of the renin-angiotensin-aldosterone system (RAAS). A comparative evaluation of pseudo-resistance and true resistance exhibited that patients with true resistance varied from pseudo-resistant patients with substantially lower BMI (p=0.02). Further, cardiovascular remodeling, lipid and carbohydrate profiles, patients with true resistance showed substantially greater levels of aldosterone (p=0.04), greater activity of oxidative stress (malonic dialdehyde, p=0.01 and diene conjugates, p=0.03), a lower level of total antioxidant protection (p=0.00), a greater level of adiponectin (p=0.00), and a lower level of leptin (p=0.00) as compared to pseudo-resistant patients in the absence of differences in BP levels.
Conclusion: Patients with resistant hypertension varied from hypertensive obese patients without resistance with greater BMI and BP, greater levels of triglycerides, insulin, HbA1c, more pronounced IR, cardiovascular remodeling, imbalance of oxidative stress – antioxidant protection system, greater proinflammatory and RAAS activity. Patients with true resistance varied from pseudo-resistant patients with substantially lower BMI, greater aldosterone levels, more proclaimed imbalance of the system of oxidative stress – antioxidant protection and less proclaimed adipokines imbalance.
Impact of 2016 ASE/EACVI recommendations on evaluation of left ventricular diastolic function and clinical outcomes in patients with diabetes and hypertension without prior adverse cardiac events
In patients with diabetes, left ventricular diastolic dysfunction (LVDD) has been shown to be more prevalent and once develop to overt heart failure, bring worse clinical outcomes, as compared to those without diabetes. The complexity of the previous 2009 ASE/EACVI algorithm makes the evaluation of diastolic function (DF) challenging. Therefore, prognostic value of LVDD estimates in clinical setting is not well established.
Foo D, et al., presented a study at ESC Congress 2020: A Digital Experience which assessed the impact of 2016 recommendations in estimates of LVDD and forecasting cardiovascular outcomes in patients with diabetes and hypertension.
A total of 111 patients with diabetes and hypertension were enrolled who attended diabetic clinic follow-up at the primary healthcare settings. All patients were clinically NYHA Class I, with no prior adverse cardiac occurrences, and showed preserved left ventricular (LV) ejection fraction on echocardiography at screening. Echocardiography was executed to acquire parameters of LV dimensions, LV volumes and LVDD. Both 2009 and 2016 algorithms were applied in DF evaluation. All patients’ were followed up at 1 year to evaluate clinical outcomes.
There were 65 (58.6%) female patients. Mean age was 59.86 (7.45); mean duration of diabetes was 10.5 (5.41). 55 (50.5%) patients showed LV hypertrophy on echocardiography. Prevalence of LVDD (14.4% vs. 55.0%) and increased LV filling pressure (9.0% vs. 26.1%) were lower in 2016 as compared to 2009 recommendations. Prevalence of indeterminate DF was 18.0% and 12.6% as per the 2016 and 2009 recommendations respectively. Concordance was fair among 2016 and 2009 recommendations (k=0.29, p< 0.001), with a reclassification rate of 45.9%. None of the patients developed MACE at 1 year from the 45 patients who were diagnosed with indeterminate and normal DF according to 2016 and LVDD with 2009 algorithms. 4 patients developed MACE at 1 year from the 12 patients diagnosed with LVDD based on both 2016 and 2009 recommendations. 2016 recommendations exhibited better efficiency in predicting MACE at 1 year (sensitivity=80.0%; specificity=88.68%) as compared with 2009 recommendations (sensitivity=80.0%; specificity= 45.28%).
Conclusion: The application of 2016 recommendations showed lower prevalence of LVDD. The 2016 criteria recognize more advanced cases and predicts superior 1-year cardiovascular outcomes. Further studies are warranted to examine the prognostic effect of these criteria.
Glucagon like peptide-1 receptor agonists and their combination with sodium-glucose cotransporter-2 inhibitors improve myocardial deformation and work index in type-2 diabetes after 12-month treatment
Ikonomidis, presented an ePoster at the ESC Congress, 2020: A digital experience which investigated the effects of insulin, glucagon like peptide-1 receptor agonists (GLP-1RA), sodium-glucose cotransporter-2 inhibitors (SGLT-2i) and their combination on LV myocardial function of T2DM patients. 160 T2DM patients (age:58±10years) were randomized to insulin (n=40), liraglutide (n=40), empagliflozin (n=40) or their combination (GLP-1RA+SGLT-2i) (n=40) as add-on to metformin. The authors measured a) global LV longitudinal strain (GLS), systolic (LongSr) and diastolic (LongSrE) strain rate, global circumferential (GCS) and radial (GRS) strain, peak twisting (pTw), peak twisting velocity (pTwVel) and peak untwisting velocity (pUtwVel), b) global myocardial global work index (GWI), global constructive (GCW) and global wasted myocardial work (GWW) derived by pressure-myocardial strain loops using speckle tracking imaging at baseline, 4 and 12 months post-treatment:
It was observed that all patients improved GLS, GCS, GRS and pUtwVel (p<0.05) at 4 and 12 months post-treatment. GLP-1RA or GLP-1RA+SGLT-2i provided a greater increase of GLS (11.5% and 13% vs. 6.8% and 2.3%), GCS (11.9% and 14.6% vs. 7.3% and 3.4%), GRS (3.8% and 4.3% vs. 2.2% and 1.6%), GWI (12.7% and 17.4% vs. 3.1% and 2%), GCW (12.3% and 15% vs. 2.2% and 7.8%) and a greater reduction of GWW (38.7% and 41.6% vs. 13.5% and 4.9%) compared with insulin or SGLT-2i, despite a similar HbA1c reduction (p<0.05 for all comparisons) at 12 months. A 2-fold reduction of pTw and a 2-fold increase of pUtwVel was observed in patients under combined treatment with GLP-1RA+SGLT-2i than those under each one regimen or insulin (p<0.05). The dual therapy demonstrated superior on measured myocardial markers in patients with LVEF<55% (p<0.05).
Conclusion: It was concluded that treatment with GLP-1RA or combination of GLP-1RA and SGLT-2i for 12 months showed a greater improvement of myocardial deformation and effective cardiac work than insulin or SGLT-2i treatment in T2DM. The combined therapy as second line was superior to either insulin, or GLP-1RA and SGLT-2i separately.
Relationship between country income, socioeconomic factors and control of cardiovascular disease risk factors in patients with type 2 Diabetes: Insights from the global DISCOVER registry
Ali Malik, presented an ePoster at the ESC Congress, 2020: A digital experience which described the association of country income and patient socioeconomic factors with risk factor control in patients with T2D. The DISCOVER registry is a 37-country, prospective, observational study of 15,983 patients with T2D enrolled between January 2016 and December 2018 at initiation of 2nd line glucose-lowering therapy and followed for 3 years.
The achievement of risk factor control I(HbA1c <7%, BP <140/90 mmHg, appropriate statin) in patients without known CV disease with sub-optimally controlled risk factors at baseline was examined at the 3 year follow-up. Data pertaining to gross national income (GNI)/capita, per World Bank report was used to stratify the countries. The author revealed that amongst 9,613 patients with T2D but without CV disease (mean age 57.2 years, 47.9% women), 83.1%, 37.5%, and 66.3% did not have optimal control of glucose, BP, and statins, respectively, at baseline. Among these, 40.8%, 55.5%, and 28.6% achieved optimal control at 3 years of follow-up. As shown in the figure substantial variability in achievement of risk factor control across countries was observed but no association of country GNI/capita on achievement of risk factor control. Patients with no insurance and public insurance were associated with lower odds of achieving HbA1c <7%.
Conclusion: The DISCOVER registry established that in patients with T2D, a substantial proportion do not achieve risk factor control even after 3 years of follow-up. The GNI/capita of the country does not explain the variability across countries in the risk factor control.