What is New in Axial Spondyloarthritis? (Win)/How to Treat Spondyloarthritis (Hot)
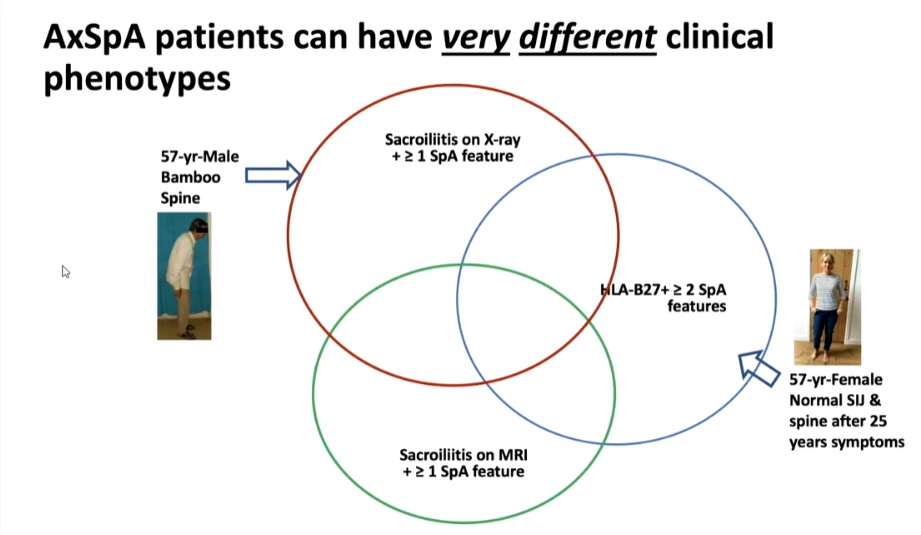
Atul Deodhar, MD presented a study “Non-radiographic or Radiographic Axial SpA: Does it Matter?” in a session at the European Alliance of Associations for Rheumatology, (EULAR) 2021, Scientific program. Population based epidemiology showed that around 1% of the US population with female predominance is ailed with Spondyloarthritis (SpA). FDA has mandated separate clinical trials will be required to approve non-radiographic SpA. Health-related quality of life (HRQOL) was found to be affected equally in both radiographic as well as non-radiographic SpAs.
From a patient’s perspective, diagnosis of non-radiographic axial SpA may be reassuring as compared to that of Ankylosing spondylitis (AS). However, from a clinician’s perspective since diagnosis is difficult, it may need re-visiting to differentiate from fibromyalgia (FM) with the help of MRIs. On the other hand, since grading of Sacroiliitis is arbitrary and has poor agreement even amongst experts, it might not make a big difference in separating them. When it comes to planning treatment, non-radiographic axial SpA with a normal MRI/CRP doesn’t respond to biologics but if the diagnosis is certain, physicians will treat it. In terms of clinical trials, FDA/EMA still differentiates AS and non-radiographic axial SpA and gives separate approval for agents, but there is an opinion that it’s about time, they start categorizing it as axial SpA. As the understanding of the pathogenesis of the disease increases, Doctors might find that they are not the same conditions and might not need drugs to prevent new bone formation. On the other hand, it has been argued that they are two ends of the same spectrum and will need the same drugs.
Axial SpA patients can have very different clinical phenotypes owing to the differential expression of genes responsible for bone destruction and new bone formation in nonradiographic axial SpA patients. Thus, there is a possibility of determining the probability of developing radiographic structural damage based on one’s genetic profile.
The Lupus Low Disease Activity State
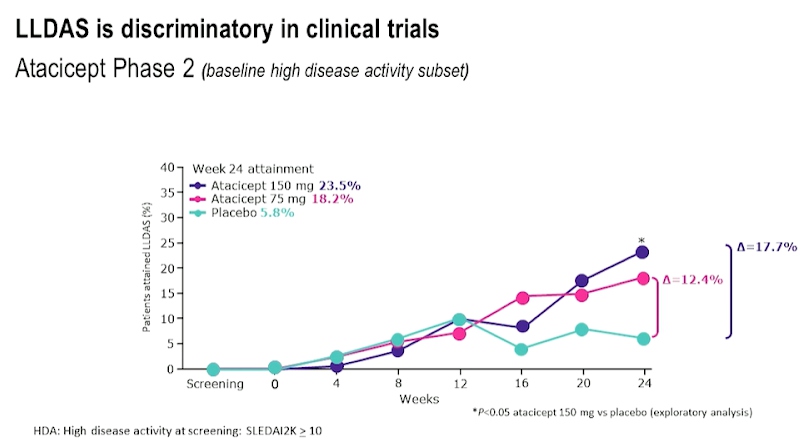
Professor Eric Morand, presented a study “Treating SLE to target : Remission or low disease activity?” in a session at the European Alliance of Associations for Rheumatology, (EULAR) 2021 Scientific program. Lupus Low Disease Activity State (LLDAS) has better performance than other definitions of low disease activity and is associated with low mortality. LLDAS has been associated with better health-related quality of life (HRQOL). As LLDAS attainment is delayed in African Americans with Nephritis, it could be dependent on genotype and phenotype. Some studies show that lower glucocorticoid dosing in LLDAS was associated with better emotional health.
LLDAS was found to be almost the same as that of remissions when SLE was treated with belimumab while comparing real world evidence. Based on post hoc clinical trial data for drugs like Anifrolumab, Azathioprine, Mycophenolate, Atacicept and Belimumab which included phase II and phase III trials in SLE treatment, it was concluded that LLDAS is discriminatory in clinical trials.
LLDAS is validated by treat-to-target (T2T) strategy endpoint for Systemic Lupus Erythematosus (SLE), that is damage, flare, health-related quality of life (HRQOL), and death. LLDAS has potential applications in clinical trials as well as in T2T strategies in practice.
Physician Global Assessment in the Definition of Targets in SLE Management: Yes or No?
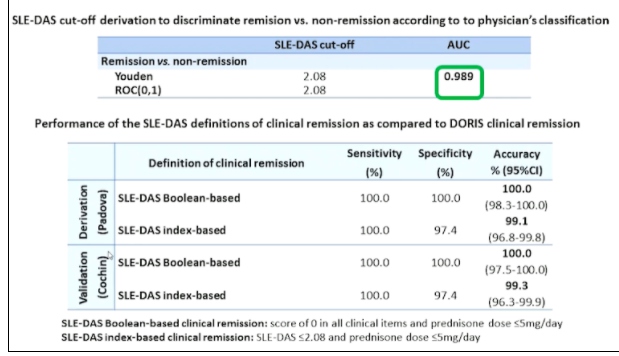
Professor Andrea Doria, presented a study “Treating SLE to target: remission or low disease activity?” in a session at the European Alliance of Associations for Rheumatology, (EULAR) 2021, Scientific program. The Physicians Global Assessment (PGA) was demonstrated to be a valid, responsive and feasible instrument but its reliability (interrater and intrarater reliability) was strongly impacted by the scale adopted suggesting the need for standardized scoring. PGA was mostly used with SLE Disease Activity Index (SELDAI) in order to complement this instrument which lacks of some manifestation or PGA is included in composite indices such as SRI, BICLA, LLDAS or DORIS remission. Systemic lupus erythematosus disease activity state (SLE-DAS) is a new instrument of 17 weighted clinical and laboratory parameters attributed to SLE and assessed at each outpatient visit. SLE-DAS enables accurate and user-friendly definitions of remission and categories of lupus disease activity.
Methodology- An observational multi-centre study was performed on SLE patients fulfilling the ACR’97 &/ or SLICC’12 classification criteria. Definition of SLE-DAS cut-off values for remission and disease activity categories were determined by ROC curves and clinical judgement by experts. Index based definition and Boolean based definitions were derived and validated for SLE-DAS in clinical remission state.
Axial SpA patients can have very different clinical phenotypes owing to the differential expression of genes responsible for bone destruction and PGA has poor reliability which derive not only by physician subjectivity but also by the variability in the assessment. Additionally, the role of PGA in assessing disease activity is limited when used as a single instrument. Adding PGA<0.5 to cSLEDAI=0 led to loss in remission in a consistent proportion of patients without increasing the performance of cSLEDAI=0 alone in identifying patients who don’t develop damage. SLE-DAS is a new disease activity instrument which enables an accurate and user-friendly definition of remission which does not need PGA.
Associations of Baseline Use of Biologic or Targeted Synthetic DMARDs with Covid-19 Severity in Rheumatoid Arthritis: Results from the Covid-19 Global Rheumatology Alliance
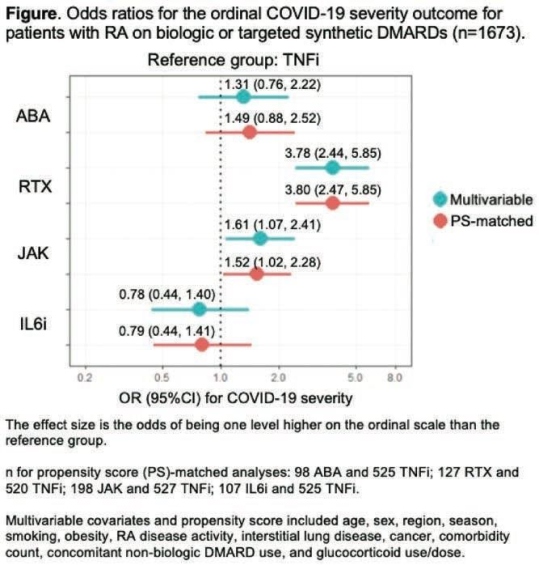
Dr Jeffrey Spark of Brigham and Women’s Hospital presented a study in plenary abstract session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. Prof Spark & colleagues used data from COVID-19 Global Rheumatology Alliance physician registry to investigate the associations of baseline use of biologic or targeted synthetic disease -modifying antirheumatic drugs (DMARDs) with a range of poor COVID-19 outcomes in rheumatoid arthritis (RA) patients. Prof Spark & his team investigated RA treated with b/tsDMARD at the clinical onset of COVID-19 (baseline): abatacept (ABA), rituximab (RTX), Janus kinase inhibitors (JAK), interleukin-6 inhibitors (IL6i), or tumor necrosis factor inhibitors (TNFi). The outcome was an ordinal scale (1-4) for COVID-19 severity: 1) no hospitalization, 2) hospitalization without oxygen need, 3) hospitalization with any oxygen need or ventilation, or 4) death.
Of the 1,673 patients with RA on b/tsDMARDs at the onset of COVID-19 154 were on ABA, 224 were on RTX, 306 were on JAK, 180 were on IL6i, and 809 were on TNFi. Overall, 498 (34.3%) were hospitalized and 112 (6.7%) died. Aer propensity score matching, RTX was strongly associated with greater odds of having a worse outcome compared to TNFi (OR 3.80, 95% CI 2.47, 5.85;). Among RTX users, 42 (18.8%) died compared to 27 (3.3%) of TNFi users. JAK use was also associated with greater odds of having a worse COVID-19 severity (OR 1.52, 95%CI 1.02, 2.28). RTX result was not a shock to the investigators. Dr Spark noted, but the association between JAK inhibitors and COVID 19 severity was, “particularly since baricitinib has shown efficacy in treating COVID-19”. ABA & IL6i, on the other hand were not associated with COVID-19 severity with TNF inhibitors. TNF inhibitors having an overall positive benefits. It could be that they are another therapeutic to consider for COVID 19. I know there are trials ongoing.
RTX or JAK was associated with worse severity of COVID-19 compared to TNFi use. The very elevated odds for poor COVID-19 outcomes in RTX users highlights the urgent need for risk mitigation strategies, such as the optimal timing of vaccination.
Is Remission Achievement Possible with the Today’s Drugs?
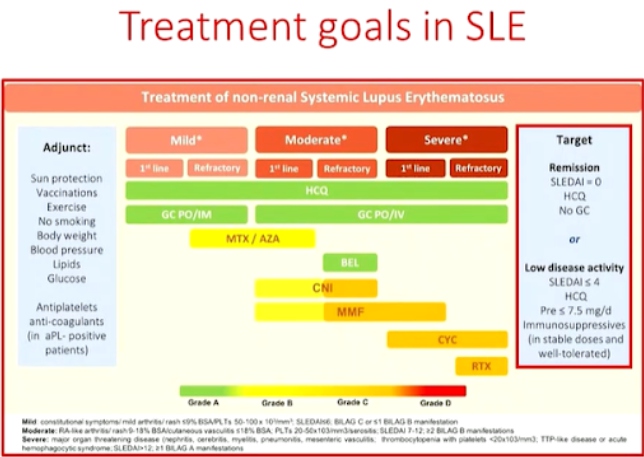
Dr. Chiara Tani presented a study “Treating SLE to target: remission or low disease activity?” in a session at the European Alliance of Associations for Rheumatology, (EULAR) 2021, Scientific program. Recent studies have shown over 50% of the patients are able to achieve at least one remission state during their follow-up visits. Prolonged remission is associated with better quality of life scores, reduced risk of cardio vascular disease and significant reduction in mortality. The longer the patients stay on remission, the less damage accrual that occurs.
Durable remission in SLE is rare, some predictors of non-remission or longer time to remission include disease activity and type of manifestations (skin and joint), therapy, ethnicity (African-American) and preexisting organ damage. The obstacle to overcome in achieving stable remission are disease flares despite therapy, persistently active disease and inability to reduce Glucocorticoids (GC). Up to 50% of the patients experience a period of persistently active disease in a given year. Muco-cutaneous (50%) and arthritis (42.9%) are the most frequent clinical persistent involvements. GC are the most useful drugs to induce rapid remission in the active disease. Other factors that contribute to flares or persistent activity include high disease activity, rare disease manifestations, poor adherence to treatment, comorbidities, & limited access to treatment.
Remission is an achievable target in the majority of SLE patients. Prolonged remission, especially off treatment is less frequent. Durable remission is prevented in a significant proportion of patients by recurrent flares, persistent disease activity or an inability to taper GC. Thus, newer more effective drugs, steroid sparing strategies, strategies to prevent poor adherence, patient empowerment, and equal access to better standard of care across different countries will help bring out drastic improvement in SLE therapy.
How to Assess Heart Function in Rheumatic Diseases?
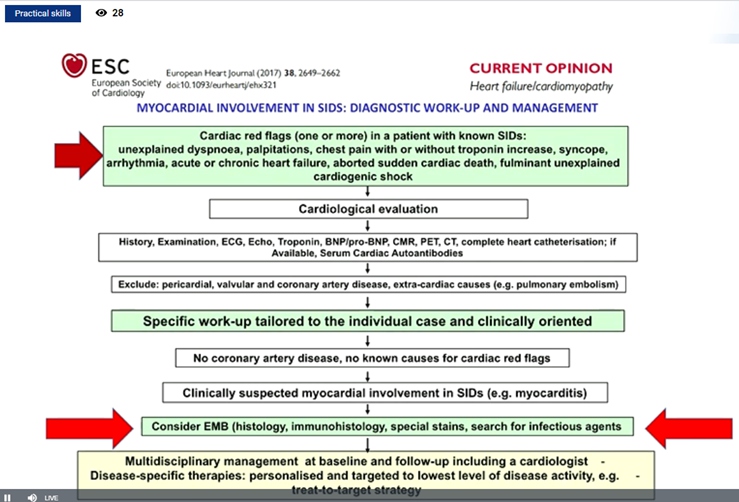
Cerinic MM, presented a study in a session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. The goal of this study was to analyze the heart function in rheumatic diseases in particularly how to assess it either in systemic sclerosis (Ssc) & rheumatic arthritis as a prototype disease.
Heart involvement kills the SSc patient silently, it is hard to detect, and when symptomatic it is too late. In 75% cases, if magnetic resonance imaging is used it may be detected as soon as possible. SSc is mainly related to inflammation which evolving fibrosis and further lead to heart failure and arrhythmia. Mayocarditis is responsible for evolution to mayocardial fibrosis. It was reported that prednisone 1mg/kg/day + azathioprine 2.5 mg/kg/day provides dramatic improvement on clinical condition with marked decrease of ventricular arrhythmic burden. Ventricular ectopic beats (VEBS)> 1190/24 hr. in SSc identifies patients at high risk of life threatening arrhythmic complications. Thus, 24 hr- ECG-Holter should be consider as an additional risk stratification test to select SSc-patients at high risk of SCD, in whom an ICD – implantation could represent a potential life-saving intervention. Similarly, echocardiographic alteration can show diastolic dysfunction in systemic sclerosis patients.
hs-cTnT and NT-proBNP concentrations are increased in patients with SSc, even in those who are free of cardiovascular risk factors. These easily obtained biomarkers may be useful for systemic evaluation and stratification of SSc patients especially to identify those at risk of pulmonary hypertension. Elevated hscTnT and NT-proBNP were associated with diffuse skin involvement and directly correlated with the skin core. Long-term survival was worse in patients with increase of both cardiac biomarkers. Silent myocardoitis may be diagnosed using the Lake Louise paper criteria in SSc patients without cardiac symptoms. Cardiovascular magnetic resonance (CMR) is a promising tool to diagnose silent myocarditis in SSc and monitor the response to immunosuppressive treatment.
Myocardial damage modifies electric conduction and functionality. The ischemic microcirculatory component is always present in SSc patients. The disturbance of the electric potentials is fundamental to suspect primary heart involvement.
Value of Patient Reported Outcome Measures in Rheumatic Diseases
Alten R, presented a study in a session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. The goal of this study was to determine the value of patient reported outcome measures in rheumatic diseases. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs (2010-2020) are as follows: (1) rheumatologist are the specialist who should primarily care for patients with RA, (2) treatment of patients with RA should aim at the best care and must be based on a shared decision between the patient and the rheumatologist and (3) RA is expensive in regards to medical costs and productivity costs, both of which should be considered by the treating rheumatologist.
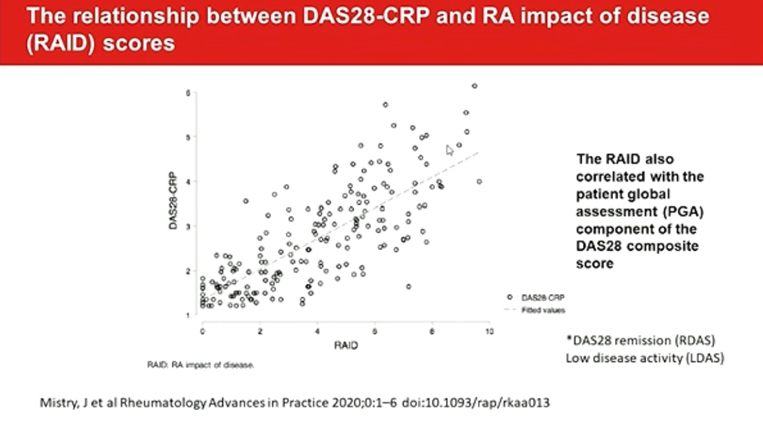
Determinants of discordance in patients and physicians rating of RA disease activity elaborate the 37% of important discordance on global assessment. Around one-third of RA patients differ from their physicians to a meaningful degree for global assessment. PROMIS can improve patient communication, decision making, satisfaction and confidence.
RAID functions well in routine care and are closely associated with subjective components of DAS28. It can easily complete by the patient at home, submitted electronically, avoiding the need for face-to face consultations. Fatigue and sleep are the worst-scoring domains in RDAS/ LDAS patients with a RAID<2. The separate use of the individual NRS of RAID (RAID.7) is valid, feasible, reliable and sensitive to change, representing an opportunity to improve the assessment and treatment of disease impact with minimal questionnaire burden. PROs ranked as most important for tracking were PROMIS Fatigue, physical function, pain intensity, pain interference, and duration of morning joint stiffness and sleep disturbances.
Since digitization and eHealth are an important part of future healthcare, combining PROs with new digital technologies could aid their uptake into clinical practice.
Is There an Added Value of Imaging to the Composite Index Measurement of Disease Activity in Patients with Rheumatoid Arthritis?
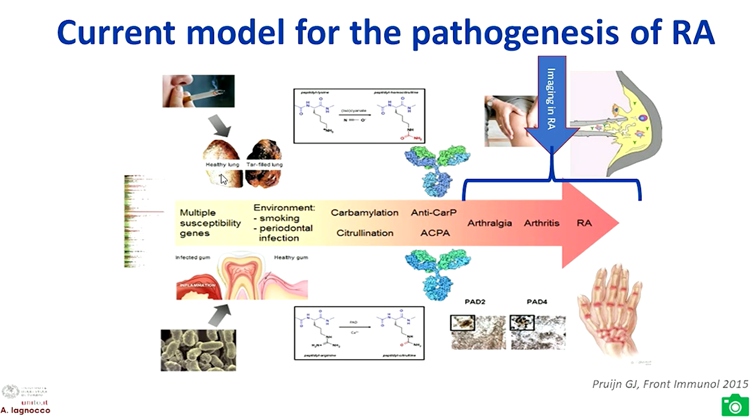
Lagnocco A, presented a study in a session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. The goal of this study was to determine whether there is an added value of imaging to the composite index measurement of disease activity in patients with rheumatoid arthritis.
Clinically, remission entails persistent absence of any sign of joint and systemic inflammation. Patientreported outcome measures (PROs) in RA includes: rheumatoid arthritis disease activity index (RADAI), RADAI-5, Patient-based disease activity score (PDAS) I and II, Patient-derived disease activity score with 28-jointcounts (Pt-DAS28), , Patient-derived simplified disease activity score with (Pt-SDAI), Global arthritis score (GAS), Patient activity score (PAS) I and II, routine assessment of patient index data (RAPID) 2-5, patient reported outcome-index (PRO-index) continuous (C) and majority (M) and patient reported outcome clinical arthritis activity (PRO-CLARA).
Advantages of MRI in RA include no radiation burden, highly sensitive for structure and inflammatory abnormities and highly predictive for development of structural lesions. While the disadvantage is it is expensive and limited availability/feasibility. Ultrasound in RA is safe, relatively inexpensive, feasible, bedside, dynamic, and superior to clinical examination and x-rays but the limitation is bone not visualized and limited acoustic windows. The findings of the TaSER study reported that in early RA, a MSUS-driven T2T strategy led to more intensive treatment but was not associated with significantly better clinical or imaging outcomes than DAS28-driven strategy.
Depending on the modality that is used, the assessment of remission by imaging encompasses the evaluation of a wide spectrum of lesions. While ultrasound is able to show intra- and peri-articular signs of inflammation and to differentiate active from inactive synovitis, MRI shows also bone marrow inflammatory related abnormalities. Moreover ultrasound detects inflammation at multi-site level and allows the measurement of stable remission over time on longitudinal evaluation.
Clinical evaluation of remission alone may be inadequate to capture a real persistent lack of inflammation at different joint sites. Imaging can complement the assessment of patients in clinical remission, offering additional data that may still reveal a certain amount of subclinical disease activity.
Treatment Of Non-biologic-dmard-ir PsA Patients with Upadacitinib Or Adalimumab Results in the Modulation of Distinct Functional Pathways: Proteomics Analysis of the Select-PsA 1 Phase 3 Study
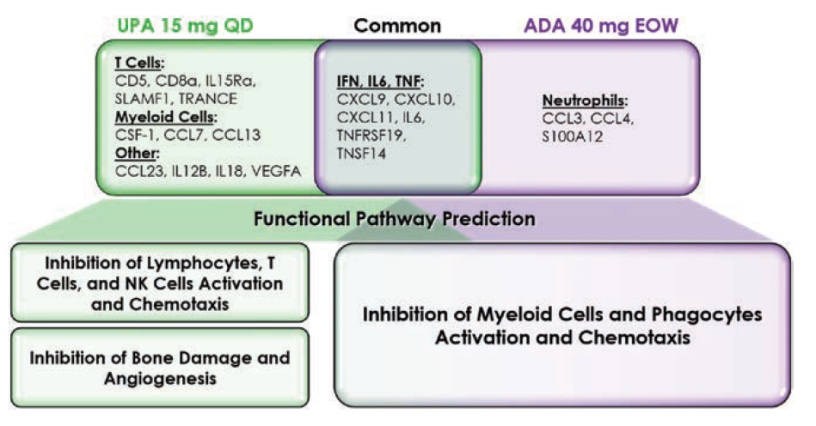
McInnes I, presented a study in a session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. The goal of this study was to examine the relative biological pathway regulation of UPA and ADA in PsA patients using a pre-defined list of plasma proteins associated with inflammation.
The patients were selected at random from the SELECT-PsA1 study (DMARD-IR PsA patients) (PBO, n=100; UPA 15 mg QD, n=100; ADA 40 mg EOW, n = 100). In plasma samples collected at baseline, week 2, and week 12, the levels of 92 inflammation-related protein biomarkers (pBM) were analysed using a multiplexed Proximity Extension Assay platform; changes from baseline in protein levels were expressed as Log2 Fold Change; a Repeated Measure Mixed Linear Model identified proteins modulated by UPA and ADA compared to Baseline. The findings were divided into three categories: adaptive immune system, innate immune system, and non-immune connective and vascular systems.
Treatment with UPA 15 mg QD resulted in distinct down modulation of T cell-associated (CD5, CD8a, IL15Ra, SLAMF1, TRANCE) and myeloid cell-associated pBM (CSF-1, CCL7, CCL13) pBM at the week 2 and 12 time periods, which was not detected in the ADA treated group. Treatment with ADA 40 mg EOW resulted in a specific down modulation of neutrophil associated pBM in a reciprocal manner (CCL3, CCL4, and S100A12). Both treatments reduced IFN-, IL6-, and TNF-related pBM (CXCL9, CXCL10, CXCL11, IL6, TNFRSF19, and TNSF14), indicating a shared node of activity linked to these critical cytokine signalling pathways. In comparison to the projected effects of ADA therapy, functional pathway modelling based on pBM data indicated that treatment with UPA is predominantly related with the suppression of T cells, but also NK cells and lymphocytes. Finally, both therapies were anticipated to inhibit various pathways involved in myeloid cell and phagocyte function.
UPA is projected to inhibit various functional pathways related with PsA pathobiology, including adaptive and innate immunity. Treatment with ADA, on the other hand, appears to influence innate immune system functioning pathways more precisely.
A Personalised Rituximab Retreatment Approach Based on Clinical and β-cell Biomarkers in Anca-Associated Vasculitis
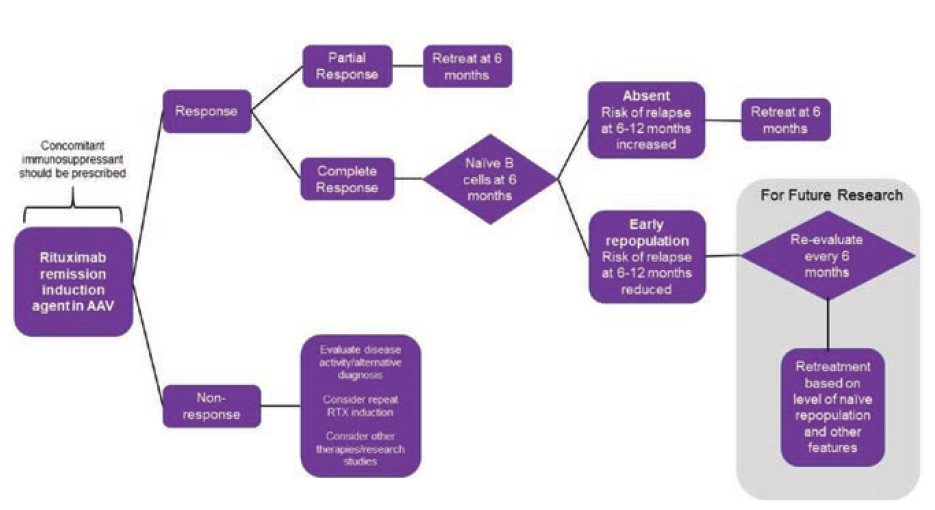
Arnold J, presented a study in a session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. The study’s purpose is to examine clinical & β-cell predictors of relapse in AAV after rituximab induction in order to establish a retreatment protocol.
60 rituximab-treated AAV patients were monitored for almost ten years in an observational analysis. Birmingham Vasculitis Activity Score v3.0 = 0 was used to identify a complete response (CR). Retreatment was offered in the event of a clinical relapse, which was defined as the appearance of new symptoms or a worsening of an existing condition (not by biomarker status). Flow cytometry was used to determine the subgroups of peripheral cells. Multivariable β Cox-Regression was used to test the predictors.
For rituximab cycles 1-5, the median time to retreatment was 87, 71, 65, 59, and 86 weeks. 137 relapses occurred in 50 patients over 417 patient-years of follow-up; 16 (in 14 patients) were major (renal=7, neurological=4, ENT=3, and respiratory=2). The rate of significant recurrence was 3.8 per 100 patient years. Concomitant immunosuppression [HR 0.48 (95% CI 0.24–0.94)], attaining CR [0.24 (0.12–0.50)], and naive β-cell repopulation at 6 months [0.43 (0.22–0.84)] were all linked to a longer time to relapse in multivariable analysis. A shorter time to relapse was related with higher baseline memory β-cells [1.01 (1.00–1.02)].
These findings imply that all patients should receive concomitant oral immunosuppressant. Those who have a partial response or no naive B-cells should be treated again after 6 months. Fixed retreatment should not be given to patients who have had a full response and nave repopulation at 6 months. This approach may help to prevent hypogamma globulinemia from occurring as a result of unneeded retreatment.
Treatment of Interstitial Lung Disease in Patients with Primary Sjogren Syndrome: A Single-center Retrospective Analysis
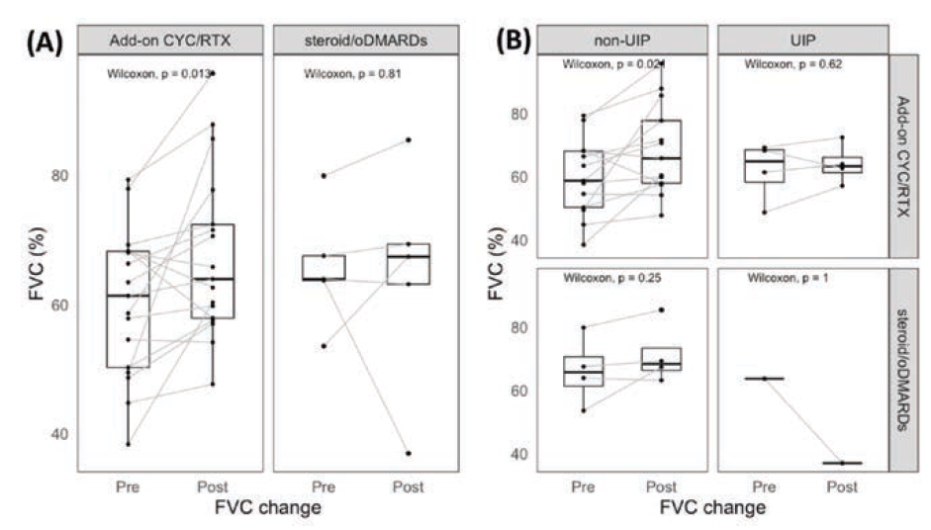
Lan T, presented a study in a session at the European congress of rheumatology (EULAR) 2021 scientific sessions: virtual congress. The aim of the study is to determine whether adding CYC or RTX (cyclophosphamide or rituximab) to steroid and oral DMARD (oDMARD) therapy improves pulmonary function test results more than standard treatment in individuals with primary sjogren syndrome- interstitial lung disease (pSS-ILD) who have poor baseline pulmonary function.
Patients with pSS-ILD were tested if they had ILD verified by a chest computed tomography. Those who had an imparied baseline pulmonary function test (FVC80% and DLCO<80%) & available pulmonary function test results at the 6th-18th month following therapy were retrospectively recruited. The patients were divided into two groups : those who received only. (steroid/oDMARD group) & those who received additional CyC and/or RTX (Add-on CyC/RTX group).
A total of 22 participants were included in this trial. These patients were 65 years old on average. 68% of them were female, and 18% of them were smokers. The median baseline FVC and DLCO were 63.5% & 54.4%, respectively. NSIP (54%) was the most common CT pattern, followed by UIP (22%), LIP (13.6%), and OP (13.6%). Five patients were given only steroid and oral DMARDs (hydroxychloroquine +/- azathioprine), whereas 17 were given CYC and/or RTX in addition (8 patients received CYC, 3 received RTX, and 6 received CYC and RTX). The FVC increased considerably from 61.3% to 65.5% (p=0.013) in the CYC/RTX group at a median follow-up time of 9.8 months (interquartile range 7.6 – 13.4 months) aer therapy, but the FVC improvement was not significant in the steroid/oDMARD group. Those with non-UIP pattern on chest CT (NSIP, LIP, and OP) displayed remarkable FVC improvement (p=0.021) in the CYC/ RTX group, compared to patients with UIP pattern on chest CT.
FVC improved considerably in individuals receiving add-on cyclophosphamide &/or rituximab treatment in pSS patients with ILD and impairment of pulmonary function at baseline (FVC< 80%, DLCO<80%).
Saving Glucocorticoids after Rituximab Treatment in Patients with Reumatoid Arthritis: Analysis of a Common Clinical Practice Cohort
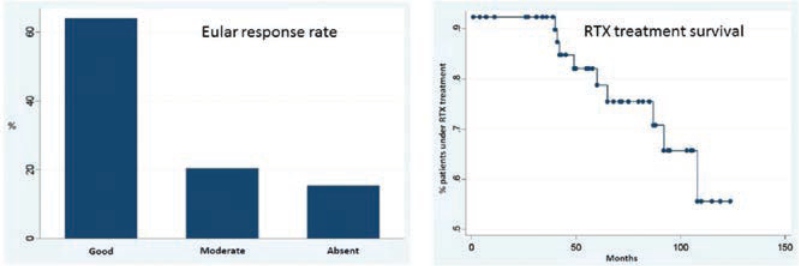
Hernández – Cruz B, presented a study in a session at the American College of Cardiology (ACC) 2021 scientific sessions: virtual congress. To investigate the long-term efficacy of rituximab (RTX) therapy & the characteristics of reumatoid arthritis (RA) patients who have been treated with the medicine since its availability in Spain.
An observational, retrospective, and analytical investigation was conducted on a group of RA patients who had received at least one dosage of RTX. From January 2007 to June 2017, the medical records of all RA patients were examined. Data on therapy (usage of RTX, related conventional synthetic disease modifying medicines [FAMEsc], dosage of corticosteroids [GC] utilised) and activity indices were obtained using a total of 178 previously specified factors. The study followed the guidelines set out by our Clinical Research Ethics Committee.
There were a total of 54 patients studied. Women comprised 74 % (n = 40) of the group, with an average age of 61.2 years (51.0 – 67.4). A total of 74% (n = 40) of the participants had a relevant comorbidity. The EULAR response rate at the end of the RTX therapy was favourable in 64% (n = 25) of the patients, with 17 (31%) of them attaining remission and a moderate response in 21% (n = 8) of them. Only 2 (4%) of patients were treated with GCC at the end of the follow-up, a significant difference from baseline (p0.00001). PDN daily doses at the end of follow-up were 6 mg in one case and 12 mg in the other, with a p=00001 difference between the two. At the end of the study, 24% of the patients (n = 13) switched or stopped taking the drug: 9 switched owing to secondary failure, 2 suspended due to side effects, 1 died owing to a preceding neoplastic process, and 1 stopped owing to complete disease remission. Survival rates were 92%, 92%, 82%, 78%, 75%, 75%, and 65% at 1, 2, 3, 4, 5, 6, and 7 years, with a mean survival rate of 90 months.
The findings suggest that patients with RA receiving RTX therapy had satisfactory disease control and drug survival rates, similar to published data. In 31 cases, RTX therapy allowed GCC therapy to be terminated (90%).

