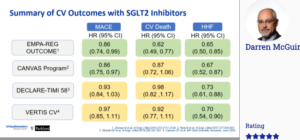
EASD/ESC Symposium: The dawn of CV risk reduction in type 2 diabetes: 5 years of SGLT2i CV outcome trials – Accumulated CV data from SGLT2i outcomes trials
McGurrie D, presented a session at the 56th EASD Annual Meeting conducted virtually, which discussed about the accumulated CV data from SGLT2i outcomes trials. Data from trials like EMPA-REG outcome, CREDENCE, DECLARE-TIMI, CANVAS program, VERTIS CV were assessed. Around 50,000 patients were included in these 6 trials and the median year of follow-up was 2.4 years. The prevalence of ASCVD at baseline varied in these trials, it was lowest 40.6% in the DECLARE-TIMI trial, and highest i.e. 100% in EMPA-REG OUTCOME and VERTIS-CV trial. Prevalence of reduced kidney function at baseline was lowest (7.4%) in the DECLARE-TIMI and highest (59.8%) in the CREDENCE trial. In summary, Dapagliflozin had a major reduction in MACE, CV death and HHF in the EMPA-REG outcome trial. In CANVAS trial, MACE and HHF achieved statistical significance whereas CV death did not achieve statistical significance. Likewise, DECLARE-TIMI and VERTIS CV trial achieved statistical significance only in reduction of HHF. In DECLARE-TIMI trial, dapagliflozin achieved significant reduction in atrial flutter and atrial fibrillation. In DAPA-HF trial, dapagliflozin reduced the risk of primary composite endpoints by 26% as compared to placebo. In the EMPEROR-REDUCED trial, Empagliflozin reduced the combined relative risk of cardiovascular death and hospitalization for heart failure by 25 % in adults with and without diabetes who had heart failure with reduced ejection fraction.
Conclusion: These 6 outcome trials of SGLT-2 inhibitors have demonstrated CV safety and incremental CV efficacy for selected outcomes. Meta-analyses results demonstrate moderate heterogeneity across the class/trials for MACE and CV death and remarkable consistency regarding effects on HHF. 2 trials have demonstrated safety and efficacy of dapagliflozin and empagliflozin for treatment of HfrEF. These results have informed product labeling modifications and guidelines/society recommendation, and have defined a new Standard of Care.
COVID-19 and type 2 diabetes: Do shared pathways have therapeutic implications?
Drucker DJ, presented a session on COVID-19 and type 2 diabetes and their shared pathways at the 56th EASD Annual Meeting conducted virtually. He summarised the latest clinical findings linking diabetes to risk of infection and differential outcomes in people with COVID-19 infection. The medications that are used to treat people with diabetes often include drugs that modulate the expression and activity of the protein ACE2, the principal receptor for SARS-CoV-2. Furthermore, observational data suggests that people treated with statins, a widely used class of cholesterol lowering drugs, may experience improved outcomes in the hospital. It is imperative to review the importance of optimising management of risk factors in people with diabetes during the pandemic, and to consider adjustment of glucose-lowering drugs in hospitalised patients with COVID-19 infection. Notably, some of the medicines, such as dexamethasone recently have been shown to be beneficial for severely ill people with COVID-19. However, dexamethasone may also worsen blood sugar control requiring careful monitoring and preferential use of insulin in COVID patients with type 2 diabetes. He will also discuss the reports of new onset type 1 diabetes and complications such as ketoacidosis in the context of SARS-CoV-2 biology, predisposition for viral infection of the endocrine pancreas, and the limitations of the available epidemiology so far. There continues to be controversy about whether the SARS-CoV-2 virus directly infects islets and insulin-producing beta cells, leading to insulin deficiency, and in some cases, new onset type 1 diabetes. Dr Drucker will highlight very new data in this area that illuminates our understanding of where the receptors for the virus are located in different cells within the pancreas.
Conclusion: It is suggested that better control of blood sugar in people with type 1 and type 2 diabetes appears to be an important modifier of COVID-19 severity, additionally emphasizing the prospect to optimise the management of diabetes in the community to mitigate the potential consequences of SARS-CoV-2 infection.
What have we learned from COVID-19 persons with type 1 diabetes?
Limbert C, presented a session at the 56th EASD Annual Meeting conducted virtually which discussed that some viruses are known to contribute to the new onset of type 1 diabetes and SARS-CoV2 infection needs to be added to this list. A population study of 23,804 COVID-19 related deaths in England during 1 March 2020 – 1 May 2020 revealed that the odds of dying in hospital with COVID-19 was higher in people with type 1 diabetes (3·5 times) compared to type 2 diabetes (2 times). However, the average age at death was 78 years in type 2 diabetes and 72 in type 1 diabetes. It appears, that in type 1 diabetes, only older people aged over 50 years), with longer duration of the disease (80% with more than 15 years of disease) and worse glucose control (glycated haemoglobin / HbA1c >10%) are at higher risk of severe clinical outcomes of COVID-19. Moreover, according to an early report by the US Centers for Disease Control and Prevention (CDC) from the United States with data from 149 082 COVID-19 cases, only 1.7% were among children <18 years. Diabetes was not among the co-morbidities, indicating that with or without diabetes, young people are coping better with COVID-19 infection. Differences in the anatomy, epidemiology and gene expressions are some of the reasons for the low prevalence of COVID-19 infection in children. In contrast to challenges related to disease severity and outcomes in type 1 diabetes, the pandemic also offered opportunities for improving diabetes care management.
Conclusion: Reports of better glycaemic control through diabetes technology among people with type 1 diabetes during the lockdown period are encouraging health care providers establishing a virtual diabetes clinic to complement standard diabetes outpatient care.
The LIBERATES Trial – improving gLucose control in patIents with diaBEtes following myocaRdial infArction: The role of a novEl glycaemic monitoring
Ajjan R, at the 56th EASD Annual Meeting conducted virtually, presented a research which evaulated the role of glycaemic monitoring in improving glucose control after heart attack in patients with diabetes. In the study, total 141 patients (102 males, 38 females) were enrolled. 72 patients were randomised to control group and 69 patients in the intervention group. Majority of patients were given antithrombotic therapy. More than 50% patients were on insulin therapy. Primary endpoint was time in range (76-90 days) and secondary endpoints were time in range (TIR) (16-30 days), hypoglycaemic exposure (16-30 and 76-90 days), HbA1c (baseline and day 90), severe hypoglycaemia, mortality and MACE and QoL. Modest increase was seen in TIR with flash monitoring compared with SMBG (17-28 mins/day) at 3 months’ post ACS but this fails to reach posterior probability cut off of 80% (59-67%) in Bayesian analysis. During mixed model analysis, TIR showed non-significant increase (48 mins/day) and significant reduction was occurred in hypoglycaemia with flash monitoring compared with SMBG at 3 months’ post ACS. On comparing flash monitoring with SMBG at 3 months, no difference in HbA1c was detected. However, the mostly similar reduction in HbA1c comparing two study arms indicates that flash monitoring may be successfully used to decrease HbA1c in T2D individuals and recent ACS with significantly lower risk of hypoglycaemia compared with SMBG. Flash glucose monitoring may result in early increase in TIR compared with SMBG in insulin treated T2D individuals with recent ACS. Reduction in hypoglycaemic exposure was evident early with flash monitoring compared with SMBG. There was a significant hypoglycaemic exposure in T2D individuals (both early and late) and recent MI who are not on insulin at baseline, which is effectively reduced by flash monitoring.
Conclusion: Flash glucose monitoring is associated with greater treatment satisfaction score compared with SMBG. Combined deaths and severe hypoglycaemic events occurred in 2 and 5 individuals in FM and SMBG arms. Preliminary data show higher MACE rate with Flash monitoring but number of event was too low and most occurred after completion of sensor wear.

Adjunctive therapies in people with type 1 diabetes -beyond insulin
Eliasson B, at the 56th EASD Annual Meeting conducted virtually, presented a research which explores additional treatments beyond insulin for people with type 1 diabetes. Type 1 diabetes is a form of diabetes in which very little or no insulin is produced by the pancreas. There is no convincing information suggesting that other mechanisms substantially contribute to the pathophysiology than beta cell destruction. As per the ADA 2020 guidelines, the risks and benefits of adjunctive agents continue to be evaluated, but only Pramlintide is approved for the treatment of type 1 diabetes. In the European commission 2019 guidelines, Forixa (Dapagliflozin) for use in type 1 diabetes as an adjunct to insulin in patients with BMI ≥27 kg/m2, when insulin alone does not provide adequate glycaemic control despite optimal insulin therapy and Sotagliflozin is not marked. Pramlintide slows gastric emptying, promotes satiety via hypothalamic receptors, inhibits glucagon secretion and regulates first-phase insulin secretion postprandially. In RCT, Pramlintide showed reduction in HbA1c, body weight and weight change. Symlin is indicated as an adjunctive treatment in adults with type 1 or type 2 diabetes who use mealtime insulin therapy and who have failed to achieve desired glucose control despite optimal insulin therapy but used in very limited numbers. In RCTs comparing Metformin and placebo, Metformin reduced BMI, insulin doses and TC. In REMOVAL trial states that long term use of Metformin in type 1 diabetes might reduce the long-term risk of CVD via small but sustained reduction in body weight and LDL-c. In RCTs comparing GLP-1RA and placebo, GLP-1RA reduced HbA1c by 0.21%, body weight and daily weight adjusted total bolus insulin dose. From DEPICT study, Dapagliflozin as adjunct therapy to adjustable insulin in patients with type 1 diabetes was well tolerated and improved glycemic control with no increase in hypoglycaemia vs placebo.
Conclusion: Dapagliflozin is currently the only approved option in EU. Metformin, GLP-1RA and SGLT2i might be considered in selected patients. However, use with great caution. In type 1 diabetes patients, non-pharmacological measures (e.g. adherence, lifestyle, CGM, insulin pump) are likely to be even more justified.

VERTIS CV outcome – Safety results, interpretation and conclusions
McGuire DK, at the 56th EASD Annual Meeting conducted virtually, presented a research which investigated VERTIS CV outcome trial in safety results, interpretation and conclusions. In comparison of VERTIS CV trial with other trials such as EMPA-REG OUTCOME, CANVAS, DECLARE TIMI, CREDENCE, VERTIS CV treatment showed lower chances of MACE and CV death. Empagliflozin in EMPA-REG OUTCOME, Canagliflozin in CANVAS, Dapagliflozin in DECLARE TIMI and Ertugliflozin in VERTIS CV, all SGLT2i showed beneficial HHF outcomes. Also, in kidney outcomes using generally consistent definitions I.e. sustained ≥40% decline in eGFR, ESKD or renal death, VERTIS CV showed superior results than other trials.
VERTIS CV provide further evidence of CV safety of SGLT2i for the treatment of patients with T2DM and adds to the evidence of benefit on HHF and kidney endpoints consistent across the class. Ertugliflozin was associated with a decrease in the risk of sustained 40% decline in eGFR with less albuminuria and with preservation of eGFR over time. VERTIS CV safety data do not materially altered estimates of risk for any specific events. Meta-analyses support contemporary society recommendations to prioritise the use of SGLT2i, independent of glucose control considerations, in patients with T2DM with or at high risk of CV and kidney complications.
Conclusion: VERTIS CV achieved its primary endpoint of non-inferiority for MACE compared with placebo in patients with T2DM and established ASCVD, demonstrating the safety of Ertugliflozin. It also demonstrated superiority of Ertugliflozin benefit on reduced risk of HHF and kidney outcomes.

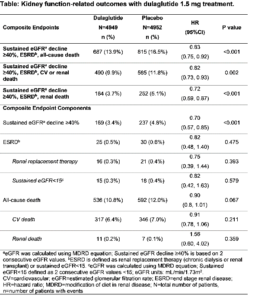
Effect of dulaglutide on kidney function-related outcomes in type 2 diabetes: post hoc analysis from the REWIND trial
In patients with type 2 diabetes (T2D) in the REWIND trial, Dulaglutide (DU) use was correlated with a decrease in the composite renal outcome for a median follow-up of 5.4 years, described as first event of new macroalbuminuria, maintained decrease in estimated glomerular filtration rate (eGFR) of ≥30%, or chronic renal replacement treatment. Shaw J, presented a post hoc analysis study at the 56th EASD Annual Meeting conducted virtually which analysed the impact of Dulaglutide on renal outcomes associated to kidney function that are typically used in renal outcomes studies, described as the composite endpoint of sustained eGFR decrease ≥40%, end-stage renal disease (ESRD), or all-cause death.
Participants with T2D and cardiovascular (CV) disease or CV risk factors were randomised (1:1) to DU 1.5 mg once-weekly or placebo. Cox proportional hazards modelling were used in this post hoc analysis for time-to-first-event to analyse the risk of renal outcomes. Two additional sensitivity analyses were held by replacing the “all-cause death” component initially with “CV or renal death” component, or “renal death only” component.
Therapy groups showed similar eGFR at baseline (mean±SD: DU=77.2±22.7; placebo=76.6±22.8). DU group showed substantially lower incidence rate of the composite endpoints than placebo with 17% risk reduction when incorporating all-cause death, 18% risk reduction when incorporating CV or renal death, and 28% risk reduction when only incorporating renal death (Table). Dulaglutide group with the substantially lower proportion of participants with sustained eGFR decrease ≥40% as compared to placebo primarily driven the impact.
Conclusion: In patients with T2D and established CV and CV risk factors, therapy with Dulaglutide 1.5 mg was correlated with a 17% risk reduction in kidney function-associated outcomes, recommending prospective delay in development of diabetic kidney disease.
GLP-1 hypersecretion in gestational diabetes
Incretins are important for a sufficient insulin secretion in reaction to ingestions of nutrients. This incretin impact is weakened in patients with diabetes, prediabetes or a specific genetic background. Data on incretin secretion and action in pregnancy are short. Fritsche L, at the 56th EASD Annual Meeting conducted virtually, presented a study which explored the incretin response with an oral glucose tolerance test (OGTT) in pregnant women with and without gestational diabetes. Pregnant women from the ongoing PREG study experienced 5-point OGTT with 75 g glucose. A plasma glucose, insulin and C-peptide were measured at all time points and total GLP-1 at minute 0, 30 and 120. Indices of insulin secretion and increase in GLP-1 were determined from the difference at min 0 and 30. A linear regression was used to assess the association of GLP-1 and glucose with insulin secretion.
163 women were examined in gestational week 26.8 (±2.0 SD), GDM was present in 30 (18.4%) women. Women with GDM showed substantially lower insulin secretion (p=0.04, adjusted for BMI, age, week of gestation, insulin sensitivity). Following glucose intake, GLP-1 levels were increased with a peak at 30 min. GLP-1 levels at minute 30 and AUCGLP-1 was substantially greater in women with GDM (by ~20%, both p=0.03, adjusted for age, BMI and week of gestation). The GLP-1 increase was correlated with insulin secretion only in GDM, however not in women with normal glucose tolerance. Even after adjustment for increase of glucose and basal insulin levels, this correlation remained substantial (GDM group: p<0.001, non GDM: p=0.69).
Conclusion: Women with GDM showed lower insulin secretion despite enhanced GLP-1 levels in OGTT. The additional pronounced GLP-1 increase in women with GDM could be part of a compensatory process preventing GLP-1 resistance. Furthermore, this phenomenon recommends a dysfunction of glucose stimulated insulin secretion to incretin resistance.
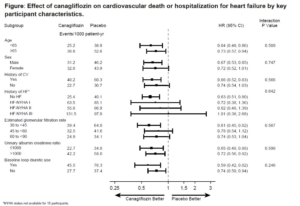
The effects of canagliflozin on heart failure and cardiovascular death by baseline participant characteristics: analysis of the CREDENCE trial
Patients of type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD) are at great risk for hospitalized heart failure (HHF) and these occurrences are decreased by Canagliflozin (CANA). Zeeuw D, presented a study at the 56th EASD Annual Meeting conducted virtually, which explored whether the impact of CANA on HHF or cardiovascular (CV) death varies by key participant characteristics. CREDENCE randomized participants with T2DM and CKD to CANA or matching placebo. In this evaluation, the impact of CANA on the prespecified secondary outcome of HHF/CV death was assessed by baseline characteristics. Hazard ratios (HRs) and 95% CIs were measured with Cox regression models, with subgroup using therapy interaction terms added to test for heterogeneity.
432 patients exhibited a HHF/CV death event from 4401 trial participants, over a median follow-up of 2.6 years. Participants those with a history of CV disease or HF, lower eGFR, greater UACR and baseline use of loop diuretics had greater risk and were included. CANA showed reduction in the risk of HHF/CV death by 31% in the overall population (HR 0.69, 95% CI 0.57, 0.83), with compatible impact over a broad range of participant subgroups incorporating those at high risk (all pinteraction>0.246; Figure). The impact of CANA on HHF alone (HR 0.61, 95% CI 0.47-0.80) was also similar over most key participant subgroups (all pinteraction>0.10).
Conclusion: CANA compatibly showed reduction in the risk of HHF/CV death and of HHF in T2DM and CKD over a broad range of participant subgroups, incorporating those with and without prior HF.
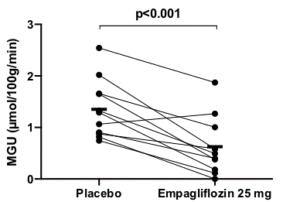
Empagliflozin reduces myocardial glucose uptake in persons with type 2 diabetes: a randomised double-blind, placebo-controlled crossover study
Sodium-glucose cotransporter 2 (SGLT2) inhibition decreases cardiovascular morbidity and mortality. SGLT2 inhibition enhances ketogenesis, which serving as a cardioprotective oxygen-sparing fuel may assist to the advantageous impact (“the thrifty substrate hypothesis”). To assess this hypothesis, Lauritsen KM, presented a study at the 56th EASD Annual Meeting conducted virtually which analysed the impact of Empagliflozin (EMPA) on cardiac glucose and free fatty acid (FFA) utilization and oxygen consumption. In a randomized double-blind, placebo-controlled crossover study, 13 individuals with type 2 diabetes (3 women; HbA1c: 57±6 mmol/mol; age: 62 (53-70) years) were treated for 4 weeks with EMPA or placebo. 24-hour blood pressure (n=13) and 48-hour continuous blood glucose (n=13) were recorded at the end of each therapy period. Cardiac glucose uptake and cardiac palmitate uptake, oxidation and esterification were determined in the postabsorptive state following an overnight fast with 18F-FDG (n=11) and 11C-Palmitate PET/CT (n=10), respectively. Myocardial oxygen consumption and myocardial external energy efficacy were determined with 11C-acetate PET/CT (n=10).
EMPA showed reduction in 48-hour mean blood glucose (8.0 ± 0.9 vs. 9.4 ± 2.2, p<0.01) and 24-hour mean arterial pressure (88±5 vs. 92±8 mmHg (p<0.05)). EMPA elevated circulating FFA (1.0±0.4 vs. 0.8±3 mmol/L (p=0.02)) and 3-hydroxybutyrate (130 ± 17 vs. 65 ± 8 μmol/L (p<0.01)) concentrations. EMPA exhibited reduction in myocardial glucose uptake (MGU) (0.6±0.6 vs. 1.4±0.6 μmol/100g/min (p<0.001) (figure 1)). EMPA did not impact myocardial FFA oxidation rate (7.2±3.1 vs. 8.0±3.1 μmol/100g/min (p=0.56)), FFA esterification rate (1.3±0.7 vs. 1.1±0.6 μmol/100g/min (p=0.34)) or total FFA uptake rate (8.4±3.6 vs. 9.1±3.2 μmol/100g/min (p=0.76). EMPA did not change myocardial external energy efficacy (29.5±7.3 vs. 27.7±4.5 % (p=0.22)) or myocardial oxygen consumption (8.97 ± 1.11 vs. 9.77 ± 1.34 ml/100g/min (p=0.12)).
Conclusion: EMPA showed reduction in postabsorptive myocardial glucose uptake by 57% however does not impact myocardial FFA utilization although substantially elevated levels of substrate in the form of circulating FFAs. EMPA therapy hence appears to selectively channel myocardial substrate utilization from glucose to other sources such as ketone bodies. But, this shift in myocardial substrate utilization does not seem to enhance either myocardial external energy efficacy or myocardial oxygen consumption.
Effects of 6 weeks of treatment with dapagliflozin, a sodium-glucose co-transporter 2 inhibitor, on myocardial function and metabolism in patients with type 2 diabetes
Oldgren J, presented a study at the 56th EASD Annual Meeting conducted virtually, presented a which aimed to investigate early impacts of Dapagliflozin (DAPA) on myocardial function and metabolism in patients with type 2 diabetes (T2D) without heart failure (HF), which could aid describe the decreased risk for HF hospitalization detected within a few months in sodium-glucose co-transporter 2 (SGLT2) inhibitor outcome trials. In a 6-week parallel group, double-blind study, T2D patients with BMI ≥25 kg/m2, with left ventricular (LV) ejection fraction >50% and without HF, on stable Metformin, and no other antidiabetic therapy, were randomized to placebo (n=26) or 10 mg/day DAPA (n=27). Analyses included cardiac MRI, [11C]-acetate positron emission tomography (PET) (oxygen consumption and perfusion) of the heart and [18F]-FTHA PET (fatty acid uptake) of the heart and liver, and analyses of circulating biomarker levels at baseline and at 6 weeks. Placebo-adjusted changes in the per-protocol analysis set were evaluated using ANCOVA as least square means with 95% confidence intervals.
Analysable patients (placebo: n=24, DAPA: n=25; 53% males) showed a mean (SD) age of 64.4 (7.2) years, BMI of 30.1 (3.7) kg/m2, HbA1c of 6.7 (0.6) %. Hypertension (75.5%) and dyslipidemia (57.1%) were frequent, while few patients showed a prior cardiovascular occurrence. At 6 weeks, DAPA group showed reduction in body weight and HbA1c vs placebo. Myocardial efficacy was not influenced, however DAPA group showed reduction from baseline in external LV work, total LV energy consumption and myocardial perfusion, however not substantially vs placebo. No substantial impacts on LV sizes or volumes were detected, while left atrial volume was decreased in patients randomized to DAPA. DAPA therapy showed reduction in Global radial strain vs placebo, while increase in global longitudinal and circumferential strain. Myocardial fatty acid uptake was not influenced; however hepatic uptake of fatty acids was elevated by DAPA vs placebo. Plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels were unaltered. No adverse events leading to study therapy discontinuation were outlined.
Conclusion: This exploratory study in patients with well-controlled T2D without HF exhibited limited impacts on myocardial fatty acid uptake, function and efficacy, however consequences specify decreased heart work following 6 weeks of therapy with Dapagliflozin.
Effect of empagliflozin on the fibrosis biomarkers and left ventricular haemodynamics in patients with type 2 diabetes and chronic heart failure
A widely used class of antihyperglycemic medications; sodium glucose co-transporter 2 inhibitor, acting on inhibiting glucose reabsorption, is exhibited advantageous in decrease of heart failure hospitalization and cardiovascular mortality. But, the processes remain unclear. Lebedev D, presented a study at the 56th EASD Annual Meeting conducted virtually, which investigated the effect of Empagliflozin on fibrosis biomarkers and left ventricular parameters in patients with type 2 diabetes mellitus (T2DM) and chronic heart failure with preserved ejection fraction (HFpEF). 35 patients with T2DM and HFpEF were enlisted in the study. Inclusion criteria were: females or males aged 40 to 75 years, glycated hemoglobin (HbA1c) 7.5-10.0%, stable antihyperglycemic therapy at least for 12 weeks. Exclusion criteria were: acute illness or infection, a cardiovascular event in the past 6 months, chronic heart failure NYHA III-IV, chronic kidney disease (estimated glomerular filtration rate (eGFR), as per CKD-EPI (eGFR < 45 mL/min/1.73 m2). Patients were acquired Empagliflozin 10 mg in 24 weeks. Transthoracic echocardiography and laboratory tests such as glycated hemoglobin (HbA1c), creatinine, galectin-3, tissue inhibitor of metalloproteinase-1 inhibitor (TIMP-1), procollagen type I carboxy-terminal propeptide (P1CP), matrix metalloproteinase-9 (MMP-9), N-terminal fragment brain natriuretic peptides (NT-pro-BNP), ST-2 were performed.
No substantial difference was detected in galectin-3, P1CP, MMP-9, ST-2 concentrations among the baseline and the end of therapy. An increase in TIMP-1 concentration was shown after 24 weeks of therapy as compared to baseline (215 ng/ml (186,5-234) versus 177 ng/ml (118,25-202,5), respectively, p=0,006). Left ventricular mass index (LVMI) substantially reduced from 126 g/m2 (95,5-154) to 111,1 g/m2 (94,8-150,0), (p = 0.043). But, this difference has become nonsubstantial after applying of Holm-Bonferroni correction. There was no substantial difference in end-diastolic volume (EDV), end-systolic volume (ESV) and end-diastolic volume index (EDVI). Positive association was detected among galectin-3 concentrations and EDV after 24 weeks of therapy (-0,532, р=0,002).
Conclusion: Empagliflozin did not influence left ventricular function in patients with T2DM and HFpEF, determined by echocardiography. Additionally, Empagliflozin therapy did not give rise to substantial changes in fibrosis biomarkers, except TIMP-1. Further research is required to clarify acquired results.


