NACMI: Outcomes From the North American COVID-19 STEMI Registry
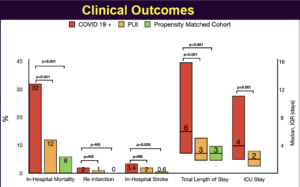
Henry TD, presented a session at the TCT Connect 2020 virtual conference, which assessed the outcomes from North American COVID-19 STEMI registry. Patients with cardiovascular risk factors or proven cardiovascular disease are more likely to have serious or critical COVID-19 disease and a primary extra-pulmonary manifestation of myocardial injury. This study was conducted to create a multi-center database of COVID+ or persons under investigation (PUI) who present with ST-Segment Elevation or new left bundle branch block (LBBB) on ECG; to compare the demographics, clinical findings, outcomes and management strategies of COVID+ patients to a propensity matched historical control of STEMI activation patients from the Midwest STEMI consortium and to develop data-driven treatment plans, guidelines and diagnostic acumen regarding these unique patients. The Society for Cardiovascular Angiography and Interventions (SCAI) and The Canadian Association of Interventional Cardiology (CAIC) in conjunction with the American College of Cardiology Interventional Council collaborated to create this multi-center observational registry, North American COVID-19 ST-Segment Elevation Myocardial Infarction (NACMI). The results observed were when compared to both PUI and propensity matched controls – ST-elevation occurred more frequently in Blacks, Hispanics and Diabetics. Cardiogenic shock (but not cardiac arrest) with lower LVEF, more atypical symptoms, and slightly higher in-hospital presentation was seen significantly in COVID+ patients with ST-elevation. 21% of COVID+ patients with ST-elevation did not receive angiography but still 71% received primary percutaneous coronary intervention (PCI). Higher in-hospital mortality and in-hospital stroke with longer length of stay was seen in COVID+ patients with ST-elevation.
Conclusion: COVID+ patients with ST-elevation represent a unique and high-risk patient population. Use of primary PCI is preferable and feasible in COVID+ patients with door to balloon times similar to PUI or COVID–patients, supporting current SCAI/ACC/AHA recommendations.
COMPARE CRUSH: A Randomized Trial of Prehospital Crushed vs Uncrushed Prasugrel in STEMI
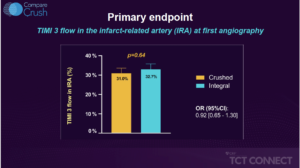
Vlachojannis GJ, presented the results of COMPARE CRUSH trial at the TCT Connect 2020 virtual conference. The COMPARE CRUSH trial was designed to test the hypothesis that patients presenting with STEMI planned for primary PCI (pPCI) will have improved markers of optimal reperfusion and clinical outcomes by prehospital administration of crushed tablets of prasugrel loading dose. Crushing tablets of the loading dose of P2Y12 inhibitors has been shown to increase bioavailability and to induce faster onset of platelet inhibition in STEMI patients. 727 patients with suspected STEMI and symptom onset within 6 hours were randomly assigned by EMS to 60 mg crushed or whole prasugrel in addition to 500 mg IV aspirin and 5,000 IU IV heparin. The independent primary endpoints were % of patients reaching thrombolysis in myocardial infarction (TIMI) flow grade 3 in the infarct-related artery at initial angiography, or achieving ≥70% ST-segment elevation resolution at 1-hour post-PCI. Secondary clinical endpoints were death, myocardial infarction, revascularization, and stent thrombosis followed up to 1 year. The results observed were 32.7% of patients achieved TIMI 3 flow in the infarct-related artery (IRA) at first angiography receiving crushed prasugrel versus 31% of patients receiving integral tablets, (p = 0.64). 59.9% of patients receiving crushed prasugrel achieved complete resolution of ST-elevation 1 hour post-PCI versus 57.3% of patients receiving integral tablets, (p = 0.55). Compared with the integral group, platelet reactivity was significantly lower at a median of 45 minutes after administration of prasugrel in the crushed group (P2Y12 reactivity units, 192 vs 227; p < 0.01). As a result, fewer patients in the crushed group had high platelet reactivity prior to the start of PCI (43.3% vs. 62.6%; p < 0.01).
Conclusion: The results of this trial indicate that crushed prasugrel did not improve TIMI 3 flow at first angiography or complete ST-segment resolution at 1 hour post-PCI compared with integral prasugrel, both of which were administered as a 60 mg load in the ambulance prior to PPCI among patients with suspected STEMI. These findings hold, in spite of the fact that crushed tablets of prasugrel lead to more potent platelet inhibition compared with integral tablets. Whether faster and more potent antiplatelet therapy can improve coronary reperfusion in contemporary STEMI treatment regimen warrants further investigation.
TICO-STEMI: A Randomized Trial of Ticagrelor Monotherapy vs Ticagrelor With Aspirin in STEMI
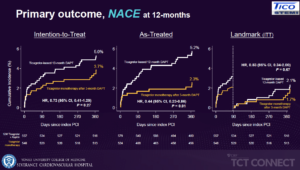
Kim BK, presented the results of TICO-STEMI trial at the TCT Connect 2020 virtual conference. The study was designed to assess the safety and feasibility of ticagrelor monotherapy after 3 months of DAPT in STEMI patients treated with ultra thin bioresorbable polymer sirolimus-eluting stents, using a prespecified subgroup analyses of the STEMI cohort of the TICO trial. TICO-STEMI trial was a prospective, randomized, multi-center trial conducted at 38 centers in South Korea. ACS patients of all types were enrolled in the study (UA, 30.3%; NSTEMI, 33.6%; and STEMI, 36.1%). Stratified randomization was performed as per the presence of STEMI. The primary outcomes were net adverse clinical event (NACE) including bleeding & ischemic outcomes, Bleeding outcomes – TIMI major bleeding, ischemic outcomes – Major adverse cardiac and cerebrovascular event (MACCE); all-cause death, MI, stent thrombosis, stroke, or TVR. Results highlighted that major bleeding risk was reduced in the ticagrelor monotherapy group and there were no significant differences observed between the two treatment groups in terms of MACCE. In the post-hoc in patients/ lesions with high risks, the risk of bleeding was decreased significantly with ticagrelor monotherapy, but there was not much difference observed in terms of MACCE. This is the first report assessing the feasibility of the ticagrelor monotherapy after short-term DAPT for STEMI patients with DES.
Conclusion: The trial results indicate that Ticagrelor monotherapy after 3-month DAPT, compared with ticagrelor-based 12-month DAPT, resulted in a reduced risk of major bleeding in patients with STEMI treated with ultrathin bioresorbable polymer sirolimus-eluting stents. As for MACCE, there were no significant differences between the two treatment groups, without significant interaction with clinical presentation in this study.
BIVALIRUDIN vs HEPARIN in Patients With Myocardial Infarction: An Individual Patient Data Pooled Analysis
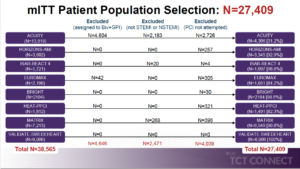
Stone GW, presented the findings of an individual patient data pooled analysis assessing bivalirudin vs heparin in patients with myocardial infarction at the TCT Connect 2020 virtual conference. Individual patient data from all 8 RCTs of bivalirudin vs. heparin in patients with MI (STEMI or NSTEMI) undergoing PCI that enrolled 1000 patients: MATRIX, VALIDATE-SWEDEHEART, EUROMAX, BRIGHT, HEAT-PPCI, ISAR-REACT 4, ACUITY, and HORIZONS-AMI were included. The pre-specified primary effectiveness endpoint included 30-day risk of all-cause mortality and the primary safety endpoint was the 30-day risk of serious bleeding (TIMI major or minor if available; alternatively, BARC type 3 or 5). From all studies, at least 1-year follow-up was included. The final study cohort included 27,409 patients (13,346 randomized to
bivalirudin and 14,063 randomized to heparin); 16,547 had STEMI and 12,152 had NSTEMI. The use of bivalirudin was associated with reductions in 30-day rates of all-cause and cardiac mortality, serious bleeding, and NACE despite increased rates of MI and stent thrombosis compared with heparin in patients with STEMI undergoing PCI. The use of bivalirudin was associated with a reduction in the 30-day rate of serious bleeding but similar rates of mortality, MI, stent thrombosis, and MACCE compared with heparin in patients with NSTEMI undergoing PCI.
Conclusion: According to this new meta-analysis, Bivalirudin is a more beneficial anticoagulation treatment for acute myocardial infarction (AMI) patients undergoing percutaneous coronary intervention (PCI) than heparin.
COMBINE (OCT–FFR): A Prospective Natural History Study Using OCT Imaging in Patients With Diabetes
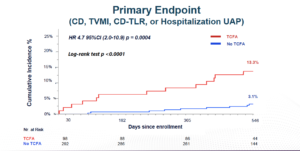
Kedhi E, presented the findings of COMBINE (OCT–FFR) trial at the TCT Connect 2020 virtual conference. COMBINE (OCT-FFR) Trial is a prospective international, natural history study. Diabetes patients underwent fractional flow reserve (FFR) assessment that had stable or acute coronary syndromes that had ≥1 (non-culprit) target lesion with a 40-80% diameter stenosis. FFR-negative patients underwent optical coherence tomography (OCT) assessment and were further medically treated. Patients were divided in two groups, depending on the presence or absence of thin-cap fibro-atheroma (TCFA). Group A included TCFA negative patients and group B included TCFA positive patients. Patients with FFR positive target lesions were revascularized, (group C). The trial aimed to show that TCFA-positive group would have a significantly higher rate of adverse events than TCFA-negative group. The primary endpoint included the incidence of target lesion related MACE defined as: cardiac death, target vessel myocardial infarction (TVMI), clinically-driven target lesion revascularisation (TLR), or hospitalisation due to unstable or progressive angina (UAP) at 18 months in the FFR-negative TCFA patients (group B) vs. FFR-negative no-TCFA patients (group A). The key secondary endpoint included incidence of MACE (Cardiac Death, TV-MI, CD-TLR, or hospitalisation for unstable angina) between FFR-negative TCFA-positive (group B) vs. Revascularized FFR-positive lesions (group C). It was observed that the incidence of primary endpoint outcomes was significantly less in the TCFA negative group (3.1%) versus the TCFA positive group (13.3%). In TCFA negative group, 0% patients had TVMI, 1.4% patients had TLR, and 1.7% patients had hospitalisation due to UAP. These primary composite endpoints were again significantly lower in the TCFA negative group. The incidence of MACE (Cardiac Death, TV-MI, CD-TLR, or hospitalisation for unstable angina) was lower in the Revascularized FFR-positive lesions vs. TCFA positive group [HR 1.25 95%CI (0.28-5.59) p = 0.77].
Conclusion: COMBINED FFR & OCT can improve the accuracy of high-risk lesion/ patient identification and should therefore be embraced.
PROSPECT ABSORB: A Randomized Trial of Interventional Treatment of Vulnerable Plaques
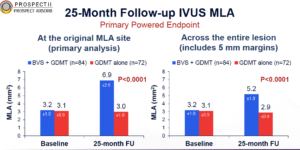
Stone GW, presented the findings of PROSPECT ABSORB trial at the TCT Connect 2020 virtual conference. This trial was designed to examine the outcomes of percutaneous coronary intervention (PCI) of non-flow-limiting vulnerable plaques. 898 patients presenting with myocardial infarction (MI) underwent three-vessel imaging with a combination intravascular ultrasound (IVUS) and near-infrared spectroscopy (NIRS) catheter after successful PCI of all flow-limiting coronary lesions. Patients with an angiographically non-obstructive stenosis not intended for PCI but with IVUS plaque burden ³ 65% were randomized to treatment of the lesion with a bioresorbable vascular scaffold (BVS) plus guideline-directed medical therapy (GDMT) vs. GDMT alone. The primary powered effectiveness endpoint was the IVUS-derived minimum lumen area (MLA) at protocol-driven 25-month follow-up. The primary effectiveness endpoint was the IVUS minimal lumen area at 25 months (powered for superiority) and the primary safety endpoint was the rate of target lesion failure at 24 months (prior to angiographic follow-up). Other important safety measures included periprocedural MACE, long-term scaffold thrombosis and randomized target lesion-related events. Angiographic follow-up at 25 months was completed in 167 patients (91.8%), and median clinical follow-up was 4.1 years. The follow-up MLA in BVS-treated lesions was 6.9±2.6 mm2 compared with 3.0±1.0 mm2 in GDMT alone-treated lesions (least square means difference 3.9 mm2, 95% CI 3.3-4.5, p<0.0001). TLF at 24 months occurred in similar rates of BVS-treated and GDMT alone-treated patients (4.3% vs. 4.5%; p=0.96). Randomized lesion-related MACE occurred in 4.3% BVS-treated patients vs. 10.7% GDMT alone-treated patients (OR 0.38, 95% CI 0.11-1.28, p=0.12).
Conclusion: The results indicate that PCI of angiographically mild lesions with large plaque burden was safe, which substantially enlarged the follow-up MLA and was associated with favorable long-term clinical outcomes. This warrants the performance of an adequately powered randomized trial to determine whether PCI treatment of focal vulnerable plaques improves patient outcomes.
PROSPECT II: A Prospective Natural History Study Using NIRS-IVUS Imaging in Patients With Acute Myocardial Infarction
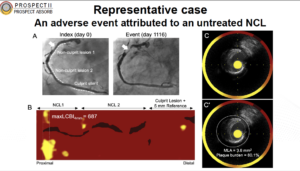
Erlinge D, presented the findings of PROSPECT II trial at the TCT Connect 2020 virtual conference. PROSPECT II was a natural history study, which used the multimodality imaging to evaluate whether NIRS-IVUS imaging can identify untreated plaques that are prone to cause future clinical events. The natural history of 805 patients who excluded randomization in PROSPECT ABSORB or who received guideline-directed medical therapy in the study were evaluated. Again, these patients all had one or more non-flow-limiting lesions with 65% plaque burden and were followed for median of 3.7 years. The primary endpoint of cardiac death, MI, or unstable angina/progressive angina requiring revascularization and/or confirmed lesion progression occurred in 13.2% of all patients at 4 years. The majority of events occurred in the untreated non-flow-limiting lesions (8.0%) while culprit-lesion MACE occurred in 4.2% of patients, a lower rate than in previous studies. On stratification of the non-culprit lesion-related MACE rates based on the NIRS-derived plaque burden, those in the upper quartile of the maximum 4-mm lipid core burden index (maxLCBI4mm ≥ 325) had a 10.1% risk of MACE compared with 4.8% among those with less plaque burden (OR 2.08; 95% CI 1.18-3.69). When high-risk plaque was defined as lesions with a plaque burden ≥ 70%, 11.0% of these patients had a non-culprit-lesion-related MACE compared with 3.6% of those with less severe plaque burden. Following treatment of flow-limiting lesions in AMI with contemporary DES, MACE occurred in 14.4% of pts at median 3.7-year FU. 8.0% were caused by unanticipated events arising from untreated non-flow-limiting plaques vs. 4.6% from recurrent events at treated culprit lesions.
Conclusion: The results indicate that 3-vessel intracoronary imaging with NIRS-IVUS was safe. Identification with NIRS of lipid-rich angiographically mild non-flow-limiting plaques was possible that could be responsible for future coronary events. The combination of lipid-rich plaque and large plaque burden identified vulnerable plaques that placed patients at especially high risk for future MACE.
Complex PCI with 1-month DAPT in High Bleeding Risk Patients: Analysis from the Onyx ONE Clear Study
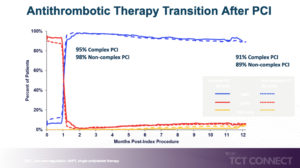
The Onyx ONE program confirmed that Resolute Onyx™ ZES is safe and effective in high bleeding risk (HBR) patients receiving 1-month dual antiplatelet therapy (DAPT) following percutaneous coronary intervention (PCI). Increasing patient and lesion complexity that is associated with higher ischemic risk shows contemporary PCI, and studies examining the benefit of longer term DAPT have demonstrated inconsistent results. Whether outcomes differ between HBR patients treated with 1-month DAPT relative to lesion complexity has not been studied. Kandzari DE presented an Onyx ONE Clear study at the TCT Connect 2020 virtual conference, which discussed the consequences of the complex PCI with 1-month DAPT in HBR patients. Onyx ONE Clear study is a prospective, multicenter, single-arm Study in which patients with high bleeding risk undergoing PCI were enrolled. It was divided into Onyx ONE RCT with Resolute Onyx™ ZES arm, with 1 Month DAPT (N=1019) and Onyx ONE US & Japan single arm study of Resolute Onyx ZES with 1 Month DAPT (n=752). Primary endpoint was cardiac death/MI assessed in “1-month clear” patients from 1-12 Months. The outcomes were compared between patients with high vs. low complexity using propensity score adjustment to adjust for baseline differences. The primary analysis population was 1506 patients with Onyx ONE Clear in which 26.6% showed Complex PCI and 73.4% had non-complex PCI by one-year. 95% patients with complex PCI and 98% patients with non-complex PCI showed significant advantage at one month of SAPT as compared to DAPT and OAC but it slowly reduced to 91% patients with complex PCI and 89% patients with non-complex PCI at 12 months. Patients with non-complex PCI showed significant reduction in cardiac death or MI and target lesion failure than patients with complex PCI.
Conclusion: A significant proportion were identified with high lesion complexity between HBR patients undergoing PCI with Resolute Onyx ZES and treated with 1-month DAPT. Absolute event rates were higher among complex patients with greater anatomic and procedural complexity; but similar outcomes were observed in HBR patients treated with complex and non-complex PCI. These data support the safety and effectiveness of 1-month DAPT among HBR patients treated with Resolute Onyx ZES irrespective of lesion complexity.
A Meta-analysis of Drug-coated Balloon Versus Drug-eluting Stent in De-novo Small Vessel Coronary Artery Disease
Revascularization of small vessel coronary artery disease (SvCAD) is correlated with high rate of adverse clinical outcomes. Drug-coated balloon is an emerging substitute to drug-eluting stent (DES) in de novo SvCAD. There is restricted data about the safety and outcomes of DCB in de-novo SvCAD. Al-abcha A, presented a meta-analysis at the TCT Connect 2020 virtual conference which compared DCB vs. DES in de novo SvCAD. The analysis included studies with at least a 1-year follow up duration from inception until April 2020. The primary outcome was All-cause mortality and secondary outcomes were myocardial infarction (MI), and target-lesion revascularization (TLR). This is the first study to include the SCAAR (Swedish Coronary Angiography and Angioplasty Registry) report following an extensive search. A total of 16,591 patients were included with 6 studies. The median-weighted follow up period was 2.8 years. 2 of the 6 studies (BELLO and BASKET-SMALL 2) were randomized clinical trials (RCTs), while the rest were observational studies. Heterogeneity was low to moderate over the trials (14-54%). There was no significant difference was observed in all-cause mortality between DCB and DES in terms of the primary outcome. There was no significant difference in the risk of MI and TLR between DCB and DES.
Conclusion: The meta-analysis showed similar clinical outcomes of DCB-treated versus DES-treated de-novo SvCAD patients. As per results, DCB can be contemplated as a reasonable therapy option for SvCAD. However, further data including large RCTs are required to confirm these results.

Comparative performance of bleeding scores in patients older than 75 years with atrial fibrillation after percutaneous coronary intervention with drug-eluting stents – The PACO-PCI Registry
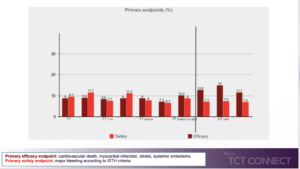
The antithrombotic therapy strategy in patients with atrial fibrillation (AF) following percutaneous coronary intervention (PCI) with drug eluting stents continues to be a subject of debate. Patients with AF and advanced age are a challenging population given the greater risk of occurrences. The evidence in the older population is restricted. de la Torre Hernandez JM, at the TCT Connect 2020 virtual conference presented a study which analysed the antithrombotic regimens used and their prognostic effect in a large population of patients older than 75 years with AF undergoing PCI with drug-eluting stents. In 20 centers, retrospective registry was conducted, including strictly consecutive patients older than 75 years with an indication for chronic oral anticoagulation for AF after PCI with drug-eluting stents. Recruitment period was from 2015 to 2019. A specific database was designed with uniform criteria for variables and events. The primary efficacy endpoint was a composite of thromboembolic events (cardiovascular death, myocardial infarction, stroke, and systemic embolisms) at 12 months after PCI. The primary safety endpoint was major bleeding according to ISTH criteria, 12 months after PCI. Total 1057 patients were enrolled in which 893 patients (84%) were given triple therapy (TT) and 164 patients (16%) were given double therapy (DT). Triple therapy significantly showed reduction in primary efficacy endpoint as compared to primary safety endpoint at one month, more than 1 month, with vitamin K antagonist (VKA), new oral anticoagulants (NOACS). However, double therapy showed reduction in primary safety endpoint as compared to primary efficacy endpoint with DKA and NOACS. PRECISE-DAPT score showed higher score for predicting risk stratification of major bleeding than HAS BLED in triple therapy group as well as in double therapy group. However, PRECISE-DAPT did not show much difference with HAS BLED in predicting NMCR bleeding in triple and double therapy group.
Conclusion: The use of triple therapy clearly predominates, in almost half of cases for more than 1 month and in half of patients with NOACs. The use of dual therapy was more commonly based on NOACs. The choice of the regimen was correlated to clinical variables, highlighting age and HAS BLED. The PRECISE-DAPT score was better predictor as compared to HAS BLED for major bleeding events either on TT or on DT. The PRECISE-DAPT score was better predictor than CHA₂DS₂-VASc for thromboembolic events in the TT group, however both were equivalent in the DT group.
Automated Classification of Computed Tomography Coronary Angiogram as Normal and Abnormal Using High Sensitivity Deep Learning Algorithm
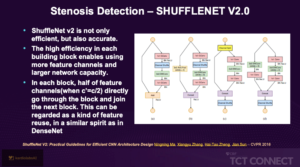
Raghav P, presented a study at the TCT Connect 2020 virtual conference, which analysed multiple Deep Learning (DL) model architecture, trained for the specific use of classifying CT Coronory angiograms (CTCA) into normal and abnormal Stenosis. This approach was compared to reporting normal CTCA studies which are superior in performance, significantly faster and less costly. 804 images from 40 Patients with a mix of normal and abnormal dataset were taken. Testing was done on deep learning model divided into stenosis to normal and abnormal and degree of stenosis. Total 812 CTCA images were enrolled in which 488 images (60%; 300 Normal, 188 Disease) were in training and 164 Images (20%; 61 disease, 103 normal) were in testing while remaining 20% was in validation. Images were in DICOM Format, which is native format for medical Imaging. Dicom Images were read using Pydicom. Multiplaner Reconstruction (MPR) for each artery was generated and saved as JPEG format. Cardiologist manually annotated each MPR image as normal or abnormal. The cleaned image was then fed to Model. The architectures of 4 widely available DL models were used for Image classification to train and test the algorithm to assess the accuracy for our data. The 4 Architecture were VGG, Shufflenet V2.0, EfficientNet and CNN. VGG showed higher specificity while Shufflenet showed more accuracy in sensitivity. EfficientNet and CNN exhibited precise results in sensitivity and specificity respectively.
Conclusion: An automated model based on Shufflenet architecture showed high accuracy for detecting CAD from CTCA. A simple automated framework was proposed which is capable of detecting the CAD from curved MPR images. This can probably be considered for primary read of the CTCAs enabling radiologists to streamline and prioritize their workload, and thereby saving time and delivering cost efficacious service. In high-risk populations, DL algorithms could also potentially be used in low -resource setting and remote areas for CAD screening. Further large-scale trials are warranted to analyse the effect of such algorithms and model architecture in daily practice.
Evaluation of Early vs. Late Benefits and Risks of Dual vs. Triple Therapy by Landmark Analysis in the RE-DUAL PCI Trial
In RE-DUAL PCI trial, dual therapy with 110 mg and 150 mg showed significant reduction in primary end point i.e. cumulative incidence of event as compared to triple therapy. While in secondary efficacy endpoint, triple therapy showed significant reduction as compared to combined dual therapy. In AUGUSTUS trial, there is an early trade off among the most severe bleeding and ischemic event composite outcomes for aspirin and placebo. Particularly, aspirin increases severe bleeding and decreases severe ischemic events by almost exactly the same amount from randomization to 30 days; this trade off is not seen from 30 days to 6 months. Peterson BE presented a study at the TCT Connect 2020 virtual conference, which evaluated early vs. late benefits and risks of dual therapy versus triple therapy by landmark analysis in the RE-DUAL PCI trial.
2725 patients with atrial fibrillation who underwent PCI were enrolled in the study. Patients were randomized to a median of 1.6 days after PCI to Dabigatran 110 mg plus a P2Y12 inhibitor, Dabigatran 150 mg plus a P2Y12 inhibitor, Warfarin plus aspirin plus a P2Y12 inhibitor. Landmark analysis was executed at 30 days and 90 days following randomization. Before 30 days, Dabigatran 110 and 150 mg dual therapy reduced the risk of major bleeding events or clinically relevant non-major bleeding events vs warfarin triple therapy. After 30 days, there was no difference in the consequences of both groups as per previous results. The net clinical benefit was significantly higher in the Dabigatran 110 mg as compared to warfarin while Dabigatran 150 mg did not show net clinical benefit as compared to warfarin after 30 days. Dabigatran 110 and 150 mg showed significant absolute risk reduction as compared to warfarin at both 30 days and after 30 days’ treatment.
Conclusion: Both doses of Dabigatran plus a P2Y12 inhibitor markedly decreased the risk of early bleeding than warfarin triple therapy. There was early net clinical benefit with both doses of Dabigatran dual therapy vs. warfarin triple therapy, without a numerical increase in thrombotic events in patients treated with Dabigatran 150 mg dual therapy. After 30 days when aspirin was discontinued, 110 mg dabigatran dual therapy continued to show lower bleeding risk than warfarin dual therapy, and 150 mg Dabigatran dual therapy exhibited similar bleeding risk profile to warfarin dual therapy. Based on this exploratory examination, either combination could be a safe substitution for warfarin when combined with a P2Y12 inhibitor, without the requirement for aspirin at any point following the doses received peri-PCI, except in select cases.


