Mondésert B, et al. Can J Cardiol. 2019 Dec;35(12):1708-1722.
Mondésert B, et al., conducted a review study to assess technological advances which are modernizing the way arrhythmias are recognized and treated in Congenital Heart Disease (CHD) patients.
Arrhythmia Detection
Ambulatory ECG monitoring
In certain participants with syncope or at high risk for atrial fibrillation (AF), the diagnostic yield of a 24-hour holter monitor is altered from 2 to 11%. By enhancing the examination duration to 7-30 days, the finding of AF or syncopal incidents increased to 16-25%. In a comparison of 24-hour Holter over 14-day examination (Zio patch, IRhythm, San Francisco, CA) in 146 patients, 61 versus 96 arrhythmias were identified (p<0.001). Implementing these examining ideas to children, 57% of noticed arrhythmias appeared following 24 hours. In alternative report, 29% of arrhythmias were identified after >48 hours.
Loop recorders
Cardiac event loop recorders are typically identified to determine if sporadic symptoms are triggered by transient arrhythmias; or whether cryptogenic stroke can be correlated to subclinical atrial arrhythmia. Where the list of suspicion for a malignant arrhythmia is high (e.g., syncope of unclear origin) but a symptom-rhythm link cannot be determined by other means, implantable cardiac monitors (ICM) can be of worth (Figure 1).
The upper panel exhibits radiographic posteroanterior and left lateral positions of an ICM in a 56- year-old patient with Turner syndrome, ventricular septal defect, and pulmonary stenosis, along with a stent in the proximal pulmonary artery. The lower panel displays a 12-second whole atrioventricular block linked with dizziness (Figure 1).
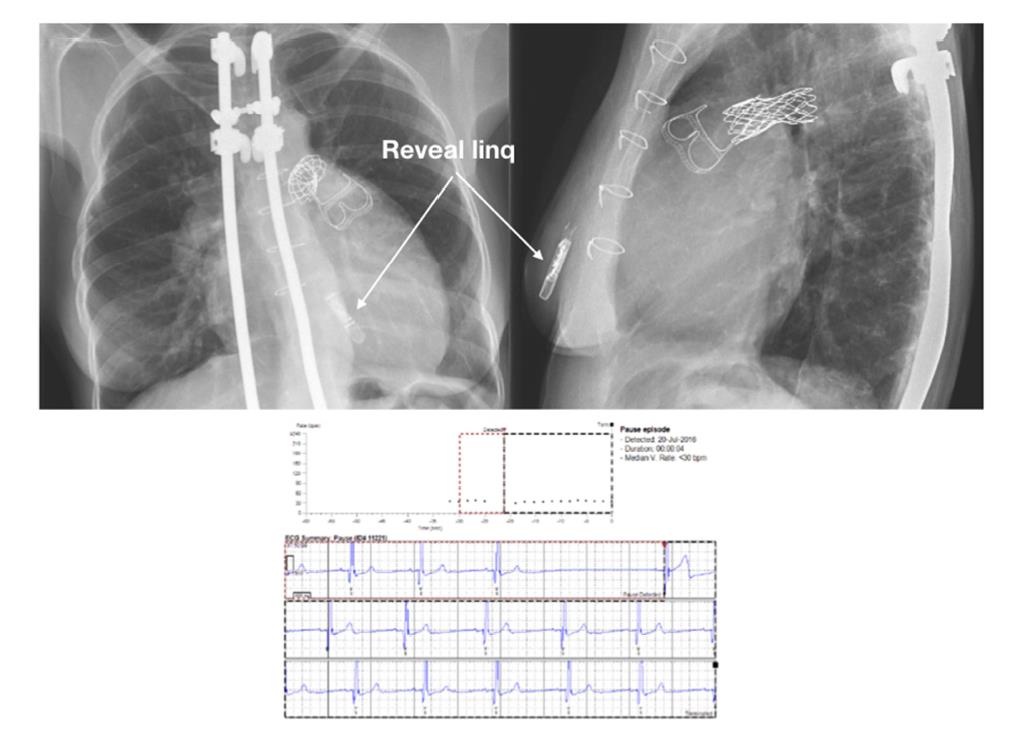
Figure 1: Implantable cardiac monitor (ICM) in an adult with CHD
Consumer wearable ECG monitoring devices
Estimating atrial arrhythmias is essential in CHD patients for various purposes which includes prevention of thromboembolic complications and heart failure, especially AF and intra-atrial re-entrant tachycardia. Growing interest was noticed in new technologies established for consumers to report and estimate patients ECG tracings with the help of smartphone applications, watches, and dedicated portable units.
In Panel A, by using the Kardia tool with an iPhone app, a 34-year-old woman with surgically restored tetralogy of Fallot captured her arrhythmia. The 30-second lead I tracing was categorized as “unspecified”. After examining it seem constant with intra-atrial re-entrant tachycardia (IART) with slow and variable atrioventricular conduction. An IART with a cycle length of 290 to 300 ms was confirmed on electrophysiological study. Shown in Panel B are surface ECG leads I, II, aVF, and VI followed by intracardiac recordings from the lateral right atrium (RA), coronary sinus (CS), and right ventricular apex (RVA). Electroanatomic mapping is represented in Panel C in a left anterior oblique view. Local activation times are color-coded, with the spot of earliest atrial activation in white and latest in dark blue (Figure 2).
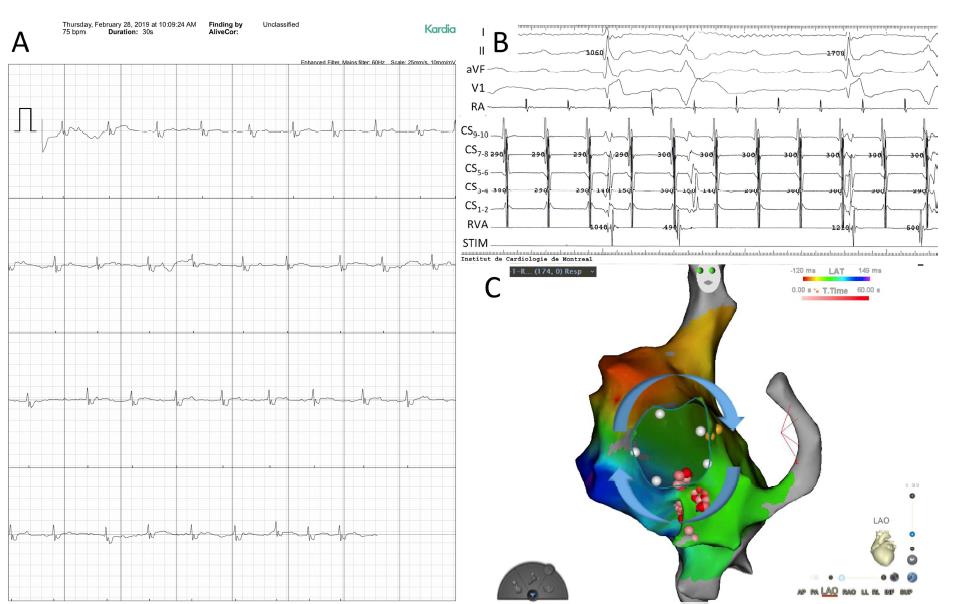
Figure 2: Arrhythmia captured by a smartphone-based application in a patient with tetralogy of Fallot
Remote magnetic-guided (RMN) catheter ablation
This system has been utilized to detect arrhythmias in pulmonary venous atria by virtue of a retrograde aortic method, therefore overcoming drawbacks to venous access and avoiding the need for complex trans-baffle punctures (Figure 3).
Posteroanterior views of electoanatomic mapping along with CT scan image integration of a non-automatic focal atrial tachycardia in a patient with a univentricular heart and extracardiac Fontan conduit are shown in figure 3. Retrograde aortic access to the pulmonary venous atrium was acquired using a soft and flexible magnetic-guided irrigated radiofrequency ablation catheter. On the left panel, the area of the focal atrial tachycardia in the inferior position of the pulmonary venous atrium is seen\, above the site where the inferior vena cava was transected and linked to the extracardiac conduit. On the right panel, the focal atrial tachycardia is spotted bordering the dense scar, which is the expected area of the transected and over-sewn inferior vena cava.
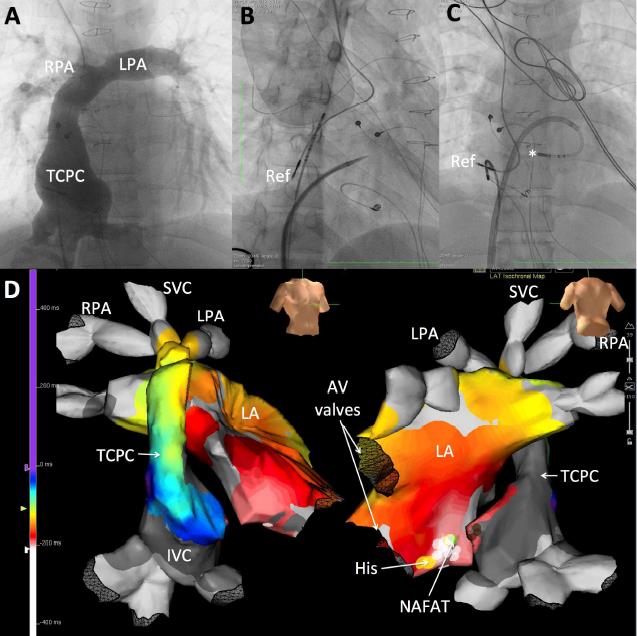
Figure 3: Remote magnetic-guided retrograde approach to catheter ablation in a patient with a single ventricle and extracardiac Fontan Catheter Ablation
Cryoablation
Cryoablation demonstrated to be safe and moderately efficient in ablating perinodal arrhythmias in patients with single ventricle physiology, congenitally balanced transposition of the great arteries, atrioventricular septal flaws, and complete exchange of the great arteries with an atrial baffle. Cryoablation was intensely successful in 75% of targeted arrhythmias, with no procedural difficulties (Figure 4).

Figure 4: Cryoablation of a para-Hisian non-automatic focal atrial tachycardia (NAFAT) through a transbaffle puncture in a patient with a double-inlet left ventricle and intracardiac lateral tunnel total cavopulmonary connection (TCPC) Fontan
Cardiac Impantable Electronic Devices
Compatibility with magnetic resonance imaging (MRI)
The majority of novel cardiac implantablle electronic devices (CIED) are now MRI conditional, which is essential to CHD patients who usually need MRIs to analyse cardiovascular and extra-cardiac manifestations of their disease.
Newer technologies
During recent decades, leadless and/or non-transvenous devices have appeared as appropriate choices in preferred patients. These devices have the potential to luckily affect complications relevant to transvenous lead systems including pericardial effusion, pneumothorax, lead dislodgement, lead fracture, venous thrombosis, stroke associated to an intracardiac shunt, pocket infection, endocarditis, and sub-pulmonary atrioventricular valve regurgitation.
S-ICD
In several aspects, CHD patients who need a defibrillator are appropriate subjects for the S-ICD by means of limited transvenous access and/or contraindications such as intra-cardiac shunts (Figure 5).
Radiographic posteroanterior (right) and left lateral (left) views of a right-sided implant of a first-generation S-ICD (1010, Cameron Health) in a 26-year-old patient with dextrocardia and a total cavopulmonary connection Fontan are shown in Figure 5. The S-ICD was implanted after a successfully resuscitated cardiac arrest. The patient had a previous epicardial pacemaker implanted for sinus node dysfunction. The leads and pacemaker were genuinely dedicated bipolar devices rendering it impossible for unipolar pacing to be programmed, which could potentially cause double counting and inadequate shock. Screening and testing were conducted with and without pacing.
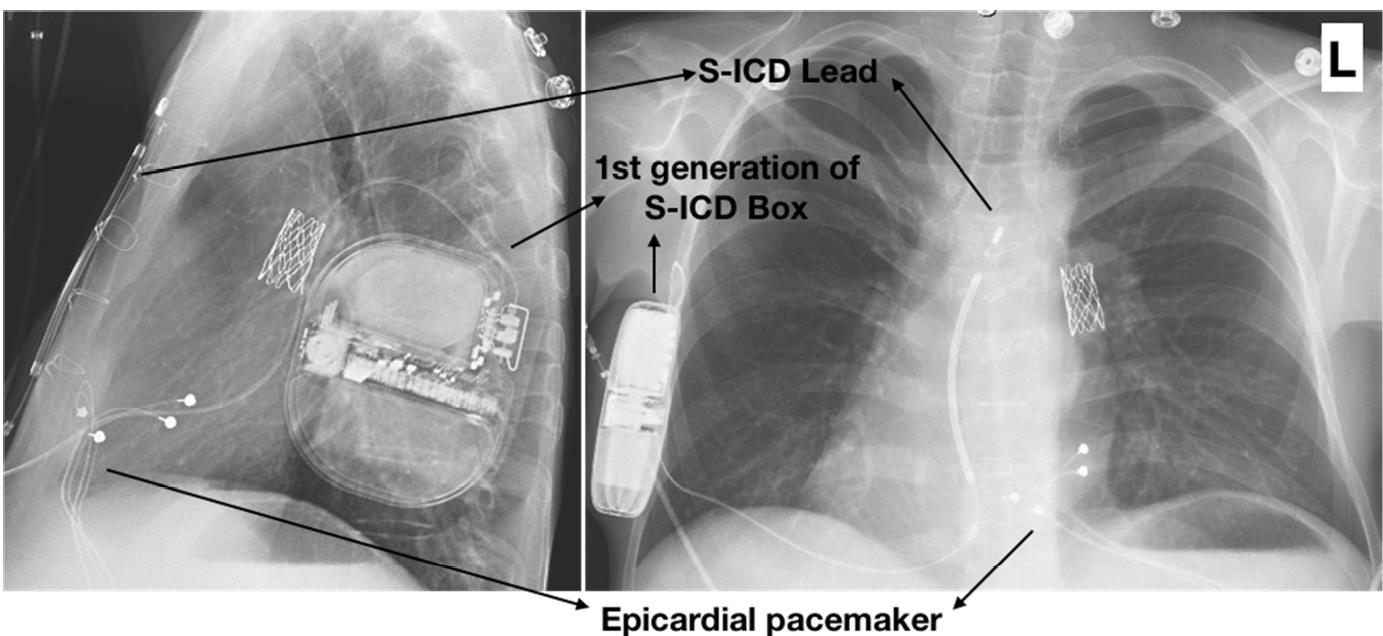
Figure 5: Subcutaneous ICD (S-ICD) in a Fontan patient with dextrocardia
Leadless pacemakers
Leadless pacemakers may be specifically entirely appropriate to CHD patients who have vascular abnormalities of the superior veins or venous obstructions as a consequence of long-standing transvenous leads (Figure 6).
Radiographic images in posteroanterior and left lateral views of a leadless pacemaker implanted in a 44-year-old patient with a partial atrioventricular septal defect, superior vena cava syndrome, and subclavian vein thrombosis following endocarditis of the tricuspid valve owing to pseudomonas aeruginosa are shown in figure 7A. A S-ICD was consequently implanted in the situation of a resuscitated cardiac arrest (figure 7B). No interaction was observed between the two devices.
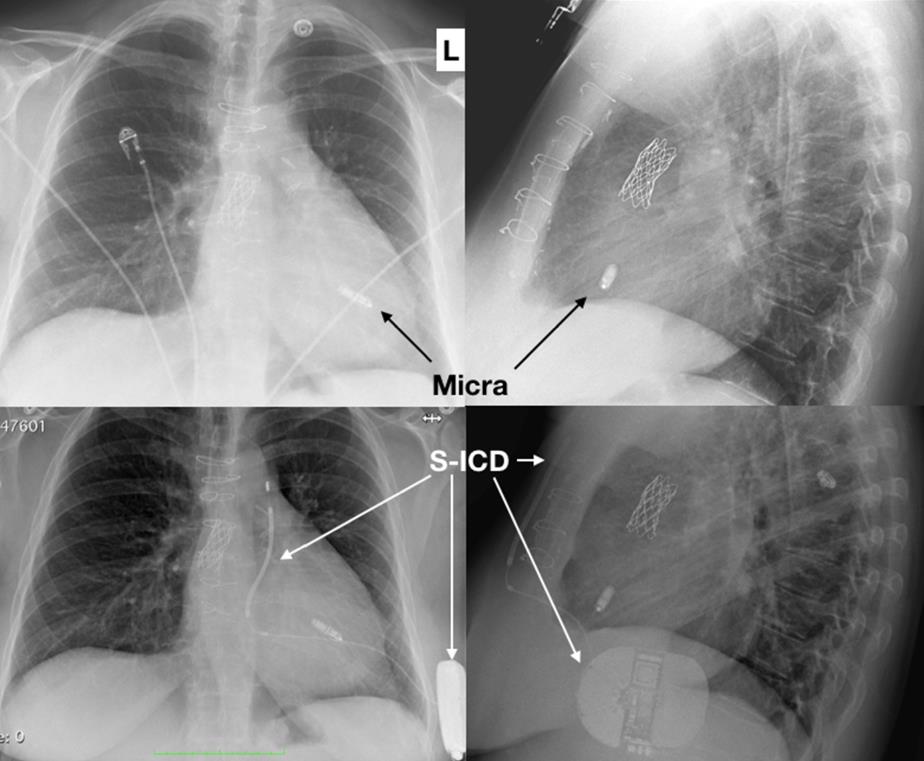
Figure 6: Leadless pacemaker (A) combined with a S-ICD (B) in an adult with CHD
Lead Extraction
As patients with CHD grows and ages, transvenous lead extraction (TLE) procedures are highly needed (Figure 7). Various evidences have established feasibility and safety in CHD patients by using a stepwise manner that uses several devices (e.g., laser, mechanical, and rotational sheaths; single or double snares) and access sites (e.g., subclavian, femoral, and jugular).
Removal of a single chamber atrial pacemaker and implantation of a dual chamber ICD was conducted in a 35-year-old with transposition of the great arteries and Mustard surgery. Angiography showed stenosis of the superior limb of the Mustard baffle, observed in the Figure 7A. The stenosis persisted despite laser lead extraction was observed in Figure 7B. Stenting of baffle is shown in Figure 7C, followed by implantation of the ICD in Figure 7D.
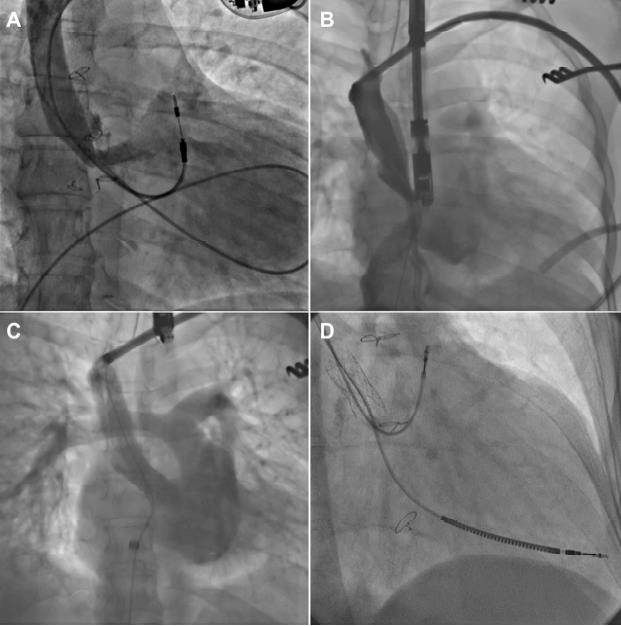
Figure 7: Lead extraction
Thus, consciousness in respect of these advances and referral to sites with particular expertise in managing arrhythmias in CHD patients may probably make a contribution to further improving finding in this extraordinary and several patient populations.

