Effects of Sodium-Glucose Cotransporter-2 Inhibitors on the Risk of Atrial Fibrillation in Older Adults with Type 2 Diabetes
Li Y. presented a study at the 83rd Scientific Sessions American Diabetes Association on June 25, 2023. In recent years, there has been growing interest in the potential protective effect of sodium-glucose cotransporter-2 inhibitors (SGLT2i) on the risk of atrial fibrillation (AF) in patients with type 2 diabetes (T2D). However, the existing clinical evidence has yielded mixed results. To address this knowledge gap, our study aimed to compare the risk of AF between SGLT2i users and users of dipeptidylpeptidase-4 inhibitors (DPP4i) and to explore the effect of SGLT2i in different subgroups.
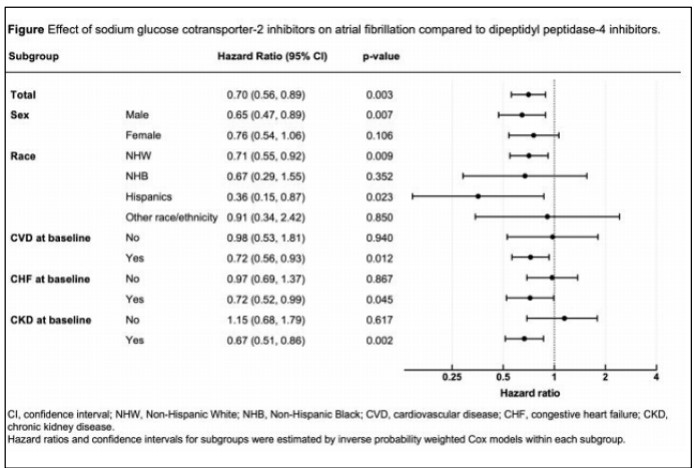
Using a 15% random sample of 2017-2018 Medicare claims data, we identified a cohort of individuals with T2D and no preexisting AF who initiated either SGLT2i or DPP4i therapy. The index date was defined as the date of the first filled prescription of either SGLT2i or DPP4i. The primary outcome of interest was the occurrence of incident AF. We employed inverse probability treatment weighting Cox regression models to analyze the data. After adjusting for confounding factors, SGLT2i use was associated with a significantly lower risk of incident AF (hazard ratio [HR] 0.72, 95% C I: 0.56-0.89) compared to DPP4i.
To conclude, the study findings suggest that SGLT2i therapy may offer a potential advantage in reducing the risk of AF in individuals with T2D, particularly among those with certain ethnic backgrounds and comorbidities.
Effects of Teneligliptin on Lipotoxicity in High-Fat Diet–Induced Diabetic Kidney Disease Model
Kim Kyuing H. presented a study at the 83rd Scientific Sessions, American Diabetes Association on June 26, 2023. Lipotoxicity, a concept central to the understanding of various metabolic disorders, has emerged as a significant contributor to the development and progression of diabetic kidney disease (DKD). In individuals with diabetes, lipotoxicity arises from multiple factors, including insulin resistance, dyslipidemia, and chronic hyperglycemia. These metabolic abnormalities disturb the delicate equilibrium between lipid uptake, storage, and oxidation in renal cells, leading to lipid overload and subsequent cellular dysfunction.
Lipotoxicity is a contributing factor in the development of diabetic kidney disease (DKD), leading to impaired function of the glomerulus and podocyte and resulting in albuminuria. While dipeptidyl peptidase-4 (DPP-4) inhibitors have shown renoprotective effects in DKD, the exact mechanism by which they protect against lipid metabolism-related DKD remains unclear. In this study, we examined the effects of teneligliptin in a high-fat diet (HFD)-induced DKD model. Male C57BL/6J mice, aged 5 weeks, were fed an HFD for 24 weeks to induce DKD.
Teneligliptin was orally administered by premixing it with the HFD for 16 weeks. At 12 weeks, body weight, blood glucose levels, and glucose and insulin tolerance were assessed, and albuminuria was evaluated at 6 and 12 weeks following teneligliptin administration. Lipid panels were analyzed, and renal cortex samples were collected for histologic examination at 16 weeks. Body weight, random and fasting blood glucose levels did not show significant differences, but total cholesterol and triglyceride levels were reduced in the teneligliptin group. The glucose and insulin tolerance tests demonstrated significant improvement in the teneligliptin group compared to the HFD group (p<0.001 and p=0.009, respectively).
Changes in albuminuria between the 6th and 12th week of the treatment period were lower in the teneligliptin group compared to the HFD group (p=0.07). Immunohistochemistry studies revealed that glomerular volume and mesangial expansion were attenuated by 26.9% and 32.15%, respectively, in the teneligliptin group (p=0.011 and p=0.043, respectively). Glomerular basement membrane thickness and foot process width were decreased by 43.2% and 9.32%, respectively, in the teneligliptin group (p=0.0007 and p=0.035, respectively).
Teneligliptin mitigated diabetic kidney disease in the HFD-induced DKD model by preserving the integrity of the glomerulus and podocyte, resulting in decreased albuminuria. Further investigations are needed to elucidate the detailed mechanisms underlying the association between DPP-4 inhibitors, lipotoxicity-induced glomerular and podocyte injury, and DKD.
Glycemic Control Assessed by Continuous Glucose Monitoring among Dialysis Patients with and without Diabetes Mellitus
Glycemic control for dialysis patients with diabetes mellitus (DM) is challenging due to limitations of HbA1c, risk of hypoglycemia, and fewer options for glucose-lowering agents. Mayeda L, presented a session at the American Diabetes Association (ADA) held in San Deigo, United States between 23rd-26th June 2023 that analyzed the glycemic patterns among dialysis patients using continuous glucose monitoring (CGM).
The Blood Sugar Sensing On Maintenance Dialysis (BLOSSOM) study is a prospective community-based cohort of people treated with dialysis, with or without DM, designed to assess the prevalence, causes, and consequences of dysglycemia. Each participant wore a Dexcom G6 CGM for 10 days. This interim analysis examined the first 153 BLOSSOM dialysis participants. Dialysis participants had a mean (SD) age of 61 (14) years, 132 (86%) underwent hemodialysis, and 21 (14%) were treated with peritoneal dialysis (PD).
The results showed that among 90 dialysis participants with DM, mean (SD) glucose management indicator (GMI) was 7.9% (1.2%), time in range (TIR) (70-180 mg/dL) was 50% (29%) and 27 (30%) participants achieved TIR ≥70%. Mean (SD) time high (>180 mg/dL), very high (>250 mg/dL), low (<70 mg/dL), and very low (<54 mg/dL) was 49% (29%), 21% (23%),
2% (11%) and 1% (11%), respectively. Of 63 dialysis participants without DM, mean (SD) GMI
was 6.2% (0.4%), and time in the normal range (70-140 mg/dL) 75% (18%). Mean (SD) time low and very low were 2% (8%) and 0% (3%), with low and very low sensor glucose values seen in 38 (60%) and 23 (37%) participants, respectively. PD patients without known DM (n=9) had a mean (SD) GMI of 6.6% (0.3%) and spent mean (SD) 54% (21%) of time in the normal range.
In this community-based sample of maintenance dialysis patients, uncontrolled hyperglycemia was highly prevalent among patients with DM. PD patients without DM were in the normal range less than half of the time. Hypoglycemia was seen in patients with and without DM. Our findings warrant additional investigation into the causes and consequences of glycemic patterns in patients on dialysis.
SURMOUNT-2 Study Finds Individuals with Type 2 Diabetes and Obesity Lost an Average of 15% of Their Body Weight when Taking Tirzepatide
Garvey at the 83rd Scientific Session of the ADA 2023 presented the findings from SURMOUNT-2, a study of tirzepatide in participants with type 2 diabetes who have obesity were announced, demonstrating more weight loss in individuals with diabetes than any other medication to date. The researchers wanted to observe how tirzepatide, a once-weekly GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) receptor agonist, affected body weight in type 2 diabetics who were overweight.
The 938 patients in the randomized trial had type 2 diabetes and were obese or overweight. The co-primary outcomes were the percentage change in body weight from randomization and the proportion of individuals who achieved a body weight reduction of at least 5% from randomization. Both objectives were monitored for 72 weeks.
The data suggest that tirzepatide may be an effective weight loss alternative for those with type 2 diabetes and obesity. After 72 weeks of therapy, participants dropped an average of 15% of their starting body weight. The total average weight loss in tirzepatide patients was 14.8 kg or 33 pounds. The HbA1c was 8% at baseline and decreased to 5.9% at the end of study. The study also found that 49% achieved a normal HbA1c below 5.7% without any severe hypoglycemia.
With the introduction of a new medicine like tirzepatide, it is evident that there is a need of a weight-centric strategy to treating type 2 diabetes when obesity is also present.
Obesity, should be treated as aggressively as other chronic diseases, and future efforts to treat obesity should focus on lowering obesity-related consequences, such as the prevention and treatment of type 2 diabetes. They also advocate for more research to see whether tirzepatide is also cardioprotective or helps to decrease adverse cardiovascular events.
ADA Presidents’ Select Abstract: CGM Initiation within Six Months of T1D Diagnosis Associated with Lower HbA1c at 3 Years
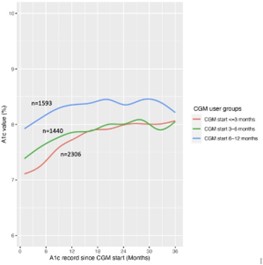
Figure 1: A1c value (%) over time based on timing on CGM initiation
Early initiation of CGM after T1D diagnosis has been associated with lower HbA1c in the years following diagnosis in single-institution studies. This multi-institution study evaluated the association between the timing of CGM initiation and HbA1c at 3 years post-diagnosis. Mann EA, presented the study results at the American Diabetes Association (ADA) held in San Deigo, United States between 23rd-26th June 2023. Data were obtained from the T1D Exchange QI Collaborative, representing 52 centers across the US. Of the 33,942 youth ≤18 years with T1D >12 months in the Collaborative data set, those who started CGM within 12 months of diagnosis were included. LOESS plots evaluated the relationship between the timing of CGM initiation and HbA1c.
Of the 5,339 youth included in this analysis, median age was 9 (IQR 6) and 26% had public health insurance. Initiation of CGM occurred within 3-months of diagnosis for 43%, between 3-6 months for 27%, and 6-12 months for 30%. There was no significant difference in CGM initiation based on insurance or the social construct of self-identified race and ethnicity, used as an indicator of systemic racism. Median HbA1c at 3 years was lower for those who initiated CGM within 3 months and between 3-6 months of diagnosis compared to those starting CGM at 6-12 months (7.3 ± 1.7 and 7.5 ± 1.7 vs. 7.9 ± 1.7; Figure 1).
Early initiation of CGM within the first 6 months of diagnosis is related to improved A1c outcomes at 3 years post-diagnosis.
Childhood Diabetes Screening Shown to Predict Future Diabetes-Related Complications
New research from the National Institutes of Health (NIH) found that blood glucose levels obtained at childhood examinations predicted future diabetes-related complications such as kidney disease (nephropathy) and eye disease (retinopathy). United States is experiencing a significant rise in obesity-induced youth-onset type 2 diabetes (T2D and hence ADA has recommended risk-based screening for prediabetes and/or diabetes in asymptomatic children with overweight or obesity by pediatric healthcare providers. However, there is currently a lack of clinical evidence for the usefulness of this screening with respect to the long-term health outcomes related to metabolic dysfunction that begins in childhood.
The study was conducted to evaluate the association of higher levels of glycemia during childhood with future microvascular complications in American Indian children – a population that is twice as likely to have diabetes than white individuals. The study findings were presented at the American Diabetes Association (ADA) held in San Deigo, United States on 23rd June 2023.
Evidence-based recommendations help drive diabetes prevention early on, and this study sheds light on how pediatric screenings are a critically important guideline. Furthermore, the findings might help inform evidence-based recommendations to ensure better care for all people with diabetes, including vulnerable communities and those at high risk. Data from a longitudinal observational study spanning more than four decades (1965-2007) within an American Indian community in the southwestern United States were utilized. The researchers examined associations of glycated hemoglobin (HbA1c) and 2-hour post-load plasma glucose (2-hr PG), obtained during childhood (ages 5-19), with future diabetes-related microvascular complications of nephropathy (albuminuria [albumin creatinine ratio (ACR) ≥ 30 mg/g], severe albuminuria [ACR ≥ 300 mg/g]), and retinopathy (at least one microaneurysm or hemorrhage or proliferative retinopathy on direct ophthalmoscopy).
Higher levels of glycated hemoglobin and 2-hr PG during childhood were significantly associated with increased risk of retinopathy. The risk of albuminuria, a symptom of kidney disease, including severe albuminuria, was also found to be elevated in children with T2D based on baseline HbA1c levels compared to those with prediabetes and normal glucose levels.
Impact of Sodium-Glucose Cotransporter 2 Inhibitors (SGLT2i) and Glucagon-Like Peptide-1 Receptor Agonists (GLP-1 RA) in COVID-19 Patients with Preexisting Type 2 Diabetes (T2D) and Cardiovascular Disease (CVD)
CVD is the leading cause of death and disability among individuals with T2D. National guidelines recommend an SGLT2i or GLP-1 RA in patients with T2D who have or are at high risk for CVD.
Nguyen C, presented a study that assessed the impact of SGLT2i or GLP-1 RA on clinical outcomes in patients with pre-existing T2D+CVD who developed COVID-19 at the American Diabetes Association (ADA) held in San Deigo, United States between 23rd-26th June 2023.
The study included adult patients with T2D+CVD who developed COVID-19 during 03/01/20- 05/31/22 and had ≥1 year of health plan enrolment before the COVID-19 diagnosis were identified from the Healthcare Integrated Research Database (HIRD®). Eligible patients were classified as guideline-concordant or not based on SGLT2i or GLP-1 RA prescription fills in the year before COVID-19 infection. COVID-19-related and all-cause hospitalization, ICU admission, and mortality were compared between SGLT2i or GLP-1 RA users and non-users. Propensity score (PS) matching and multivariable analyses were performed.
A total of 42,646 COVID-19 patients with T2D+CVD were identified with a mean follow-up of 8.6 months. The use of SGLT2i or GLP-1 RA was low (n=8,498; 20%). Patients receiving SGLT2i or GLP-1 RA were younger, had fewer comorbidities, took more CVD prevention medications, and were less likely to be hospitalized during the year before COVID-19 infection than non- users. After PS matching and multivariable adjustment, T2D+CVD patients with COVID-19 who received SGLT2i or GLP-1 RA were 12% less likely to be hospitalized and 10% less likely to be admitted to the ICU due to COVID-19 compared to those who did not (n=8,479 each group; both p<0.05). Reductions were also observed for all-cause hospitalization (6%), ICU admission (7%), and mortality (10%), but the results were not statistically significant.
The findings suggest that SGLT2i and GLP-1 RA may improve outcomes in T2D+CVD patients with COVID-19 and support existing national guidelines.

