Teneligliptin Observational Retrospective Real-World Study to Evaluate Its Effect on Renal Parameters and Safety in Indian Type 2 Diabetes Mellitus Patients with Chronic Kidney Disease (TOP RENAL)
Barkate H, presented a study at the 83rd Scientific Sessions, American Diabetes Association on June 24, 2023. Teneligliptin has been approved in India since 2015 and is widely prescribed as a dipeptidyl peptidase-4 inhibitor (DPP4i). However, there is a lack of evidence regarding its effects on renal parameters and safety in Indian patients with type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD). Therefore, this real-world study aimed to evaluate the effects of teneligliptin on renal parameters and its safety profile in this specific patient population in India.
The TOP RENAL real-world study included patients with T2DM and an estimated glomerular filtration rate (eGFR) ranging from ≤60 to ≥15 ml/min/1.73m². These patients were initiated on a daily dose of teneligliptin (20/40 mg) and continued the treatment for six months. Patients who were prescribed any other antidiabetic medication in addition to teneligliptin or experienced a change in their antidiabetic prescription during the six-month period were excluded from the analysis.
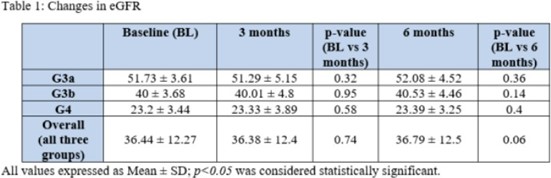
Data from 377 patients were collected between August 2022 and October 2022. The patients were grouped based on their baseline eGFR levels: G3a (eGFR: 45-59 ml/min/1.73m², n=102), G3b (eGFR: 30-44 ml/min/1.73m², n=124), and G4 (eGFR: 15-29 ml/min/1.73m², n=151). The mean age of the patients was 59.86±9.58 years, and 47% of the participants were female. Renal parameters, including eGFR, serum creatinine, and blood urea nitrogen (BUN), were assessed at baseline, 3 months, and 6 months. The results showed non-significant changes in eGFR, serum creatinine, and BUN at both the 3-month and 6-month time points across all three patient groups (Table 1). No safety concerns or hypoglycemic events were reported during the study period.
The initiation of teneligliptin in Indian patients with T2DM and mild to severe renal impairment did not raise any safety concerns and was well-tolerated. This study provides real-world evidence supporting the safety profile of teneligliptin in this specific patient population.
Diabetic Kidney Disease (DKD) Worsens CV Outcomes after First-Ever Major Cardiovascular Event in Type 2 Diabetes (T2D)
In T2D patients, cardiovascular (CV) prognosis worsens after the first CV event. Garofolo M presented a session at the American Diabetes Association (ADA) held in San Deigo, United States between 23rd-26th June 2023 that discussed the impact of DKD on mortality and subsequent CV events after a first CV event.
The death rate was recorded over a median 14.1 (IQR 13.8-14.5) years follow-up in 961 T2D subjects. Vital status (Italian Health Card Database) was censored on December 31, 2017 (major CV events available for 947 participants). The effect of DKD (ACR <30/≥30mg/g) and/or CKD-EPI eGFR ≥60/<60 ml/min/1.73 m) was determined by Kaplan-Meier (K-M) curves and hazard ratios (HR, 95% CI) by unadjusted and adjusted Cox regression models (age, diabetes duration (DD), sex, smoking, retinopathy, hypertension, dyslipidemia, prior CVD; no-DKD as reference).
The results were at census 229 subjects were dead (23.8%) and 298 participants out of 947 (31.5%) had a first CV event during a median 13.8-year pre-event follow-up. Compared with free-CV event subjects, those with CVD were older, more often men, with longer diabetes duration, worse CV risk profile, more microvascular complications, and CVD before recruitment. During a median 6 yr post-first CV event follow-up 99 subjects died: 57 (27.4%) no DKD, 42 (46.7%) DKD (K-M, p=0.005). Compared to no DKD the latter had an unadjusted HR of 1.76 (1.18-2.63, p=0.005; adjusted 1.69, 1.13-2.52, p=0.011). The death rate increased with DKD worsening with statistically significant HR (unadjusted 3.31, 1.73-6.31, p<0.0001; adjusted 3.32, 1.65-6.64, p=0.001) for ACR≥30/eGFR<60. During a median 3.1 post-first CV event follow-up, a new CV event occurred in 124 subjects: 82 (39.4%) with no DKD and 42 (46.7%) DKD (K-M, p=0.019) with unadjusted HR for a new CV event in DKD of 1.40 (0.96-2.03, p=0.077). The rate of a secondary CV event increased across DKD phenotypes with unadjusted (HR 3.42,95% CI 1.76-6.65, p<0.0001) and adjusted HR of 3.18 (1.57-6.44; p=0.001) for ACR≥30/eGFR<60, respectively.
In T2D subjects, DKD still worsens the risk of mortality and secondary CV events after the occurrence of a first CV event.
Tg/HDL Ratio and Liver Fibrosis in Diabetic Patients at an Indian Tertiary Care Center
Chandra A. presented a study at the 83rd Scientific Sessions American Diabetic Association on June 25, 2023. According to the most recent International Diabetes Federation (IDF) atlas, the global prevalence of diabetes is estimated to be 537 million individuals. In India, diabetes is a growing concern, with increased central adiposity and insulin resistance among the population. Diabetes patients are more susceptible to developing Nonalcoholic Fatty Liver Disease (NAFLD) due to their high insulin resistance. The TG/HDL ratio, an indicator of insulin resistance, is becoming increasingly sensitive in identifying individuals at risk. Measuring liver stiffness can help diabetics predict the progression of fibrosis. This study aims to evaluate the TG/HDL ratio and liver stiffness using fibro scans in diabetic patients.
A total of 181 newly diagnosed diabetics, who provided consent, underwent fibro scans to measure liver stiffness. Participants who had already received thiazolidinedione treatment or lipid-lowering therapy were excluded from the study.
The median stiffness of liver measurements ranged from 2.3 kpa to 34.8 kpa, with a median value of 8.0 kpa. The median TG/HDL ratio ranged from 0.5 to 29.3, with a median value of
4.5. A correlation of 0.2 was observed between the median stiffness and the TG/HDL ratio. Among the participants, 26.5% had liver stiffness measurements (LSM) greater than 9.1, with 23.7% of male subjects and 30% of female subjects showing advanced fibrosis. Both men and women with LSM greater than 9.1 had high TG/HDL ratios, with a mean value of 4.9 in both groups. These values were significantly higher than the TG/HDL cut-offs of 0.9 for females and 1.2 for males, which are used to predict insulin resistance.
Previous studies have linked the TG/HDL ratio and NAFLD using ultrasonography in nondiabetic individuals. However, the fibro scan is a more precise and sensitive method for screening NAFLD. Median liver stiffness serves as a predictor of liver fibrosis.
In conclusion, the study highlights a high incidence of advanced fibrosis, which is associated with elevated TG/HDL ratios. Both male and female individuals with advanced fibrosis had high TG/HDL ratios.
Cholesterol Lowering Drug Shown to Cut Major Heart-Related Events and Risk of Death from Heart Disease by One-Third for Statin-Intolerant Patients
Nissen SE at the 83rd Scientific Session of the ADA 2023 presented a session on “Cholesterol Lowering Drug Shown to Cut Major Heart-Related Events and Risk of Death from Heart Disease by One-Third for Statin-Intolerant Patients.”
According to several research, statin usage raises blood sugar because the medication prevents insulin from correctly processing blood sugar. As a result, persons may be at an increased risk of acquiring type 2 diabetes. The CLEAR Outcomes study included 13,970 statin-intolerant individuals in a masked, randomized trial. This research included 4,206 participants with heart disease risk factors but no past heart-related incident (primary prevention). The participants’ average age was 68 years, 67% had diabetes, and 59% were female. The primary efficacy outcome was a combination of cardiovascular mortality, nonfatal MI, nonfatal stroke, and coronary revascularization. The medication bempedoic acid reduced LDL-C by 22% in the trial.
Treatment for 40 months was associated with a significant risk reduction for the primary endpoint, 111 (5.3%) versus 161 events (7.6%), adjusted hazard ratio (HR) 0.70, 95% CI 0.55-0.89, p=0.002. This represents a 30% reduction in major heart-related events. A 39% reduction in the risk of death from heart disease was also observed.
These findings should serve as a wake-up call to the clinical community that patients with coronary risk factors and high cholesterol, particularly those with diabetes, should be treated with a cholesterol-lowering medicine. Bempedoic acid is a new nonstatin medicine that is now approved by the FDA for two groups of patients: those with heterozygous familial hypercholesterolemia and those with atherosclerotic cardiovascular disease.
Bempedoic acid is also the first adenosine triphosphate-citrate lyase (ACL) inhibitor approved for use as an LDL-C lowering treatment, and it operates by reducing cholesterol manufacture in the same mechanism as statins. The authors of this study state that more research is needed to determine the function of bempedoic acid in cholesterol-lowering vs anti-inflammatory effects.
Late Breaking Weight Loss Innovations: New Drug Therapies Shown to Offer Positive Outcomes for Obesity and Type 2 Diabetes Management
Gabbay R, at the 83rd Scientific Session of the ADA 2023 presented a session on “Late Breaking Weight Loss Innovations: New Drug Therapies Shown to Offer Positive Outcomes for Obesity and Type 2 Diabetes Management.” Survodutide (also known as BI 456906), a new dual glucagon receptor (GCGR) and glucagon-like peptide 1 receptor (GLP-1R) agonist, exhibited up to 18.7% weight loss in overweight or obese persons during a 46-week period in a phase 2 clinical study. The 46-week randomized, double-blind, placebo-controlled trial enrolled 387 participants with a BMI of 27 kg/m2 or higher who were randomly assigned to receive weekly subcutaneous injections of survodutide at varying doses (0.6 mg, 2.4 mg, 3.6 mg, or 4.8 mg) or a placebo. A 20-week rapidly, biweekly dosage escalation phase was followed by a 26-week maintenance phase in the trial.
The primary endpoint was the percentage change in body weight from baseline at week 46, and secondary endpoints included the proportion of participants achieving at least 5%, 10%, or 15% weight loss from baseline at week 46. The results of the study demonstrated a clear dose-response relationship, with increasing doses of survodutide correlating with greater reductions in body weight. At week 46, participants in the survodutide group achieved substantial weight loss compared to the placebo group. The mean body weight reductions were as follows: 0.6 mg (-6.2%), 2.4 mg (-12.5%), 3.6 mg (-13.2%), and 4.8 mg (-14.9%), compared to -2.8% in the placebo group. At 46 weeks, patients reaching and staying on 4.8 mg survodutide achieved a weight loss of 18.7%. Notably, 82.8% of participants in the 4.8 mg BI 456906 group achieved a weight loss of at least 5% at week 46, compared to only 25.9% in the placebo group. Survodutide’s safety profile was also investigated in the study. Adverse events were reported by 90.9% of survodutide group participants, with the majority being gastrointestinal in character. In the placebo group, 75.3% of individuals experienced adverse events. During the 46-week research period, no unanticipated safety concerns were identified. The incidence of serious adverse events was equivalent in the survodutide (4.2%) and placebo (6.5%) groups.
Survodutide demonstrates up to 18.7% weight loss in overweight or obese persons with no added safety concerns.
American Diabetes Association Releases a Guideline Update in NAFLD (Non- Alcoholic Fatty Liver Disease) and Diabetes
Liver disease affects up to 70% of people with type 2 diabetes. Nonalcoholic fatty liver disease (NAFLD), which includes nonalcoholic steatohepatitis (NASH), is the most common form of liver disease in people with diabetes. NAFLD can lead to cirrhosis and liver cancer and is associated with an increased risk of cardiovascular disease and death.
New guidelines are released and were presented at the American Diabetes Association (ADA) held in San Deigo, United States on 26th June 2023 which include recommendations for the detection and management of nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH) in people with diabetes.
The American Diabetes Association (ADA) published updates in the Standards of Care in Diabetes—2023 (Standards of Care) based on the latest scientific research and clinical trials. Section 4, “Comprehensive Medical Evaluation and Assessment of Comorbidities,” of the Standards of Care has been updated to include several new recommendations regarding screening and treatment of NAFLD/NASH in individuals with diabetes.
This update emphasizes the importance of early detection of NAFLD in people with diabetes as well as appropriate management modalities. Early detection allows for timely treatment, reducing the chance of developing other serious complications.
New Study Shows a Coordinated Care Approach Significantly Improves Quality of Care for Patients with Type 2 Diabetes and Heart Disease
In the United States alone, up to two-thirds of patients with T2D develop ASCVD in their lifetime. While ASCVD is associated with worse health outcomes in patients with diabetes compared to the general population, evidence-based therapies to reduce heart disease risk in adults with T2D are underused in clinical practice.
The COORDINATE-Diabetes trial was evaluated if a coordinated, multifaceted intervention of assessment, education, and feedback versus usual care on adults with T2D would impact the prescription of three recommended evidence-based therapies, designed to help treat patients with both diseases. This trial was presented as a late-breaking symposium at the American Diabetes Association (ADA) held in San Deigo, United States between on 26th June 2023.
The randomized clinical trial included 43 cardiology clinics across the U.S. The clinics enrolled 1,049 participants (459 at 20 intervention clinics and 590 at 23 usual care clinics) with T2D and ASCVD not already taking all three groups of evidence-based therapies, including high- intensity statins, angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), and sodium-glucose cotransporter 2 (SGLT2) inhibitors and/or glucagon-like peptide 1 receptor agonists (GLP-1Ras). The primary outcome was the proportion of participants prescribed all 3 groups of recommended therapies at 6-12 months after enrollment. The findings showed that coordinated care intervention can significantly improve the quality of care that high-risk patients receive. At the last follow-up visit, those in the intervention arm were 4.38-fold more likely to be prescribed all three recommended classes vs. the standard care arm. A total of 37.9% of those in the intervention arm had been prescribed all three classes vs. 14.5% in the standard care arm. In particular, those in the intervention arm were more than 3-fold more likely to be prescribed an SGLT2 inhibitor and/or GLP-1RA. While the study was not designed or powered to detect differences in clinical outcomes, 23 of 457 participants (5%) in the intervention group vs 40 of 588 participants (6.8%) in the usual care group experienced the composite outcome of all-cause death or hospitalization for myocardial infarction, stroke, decompensated heart failure, or urgent revascularization (21% decrease in relative risk, not statistically significant.
The study shows that by providing multifaceted interventions such as assessing local barriers and coordinating across clinicians and clinics, we can help increase the prescriptions of the therapies proven effective for patients with both type 2 diabetes and ASCVD.

