Bohm M, et al. European Journal of Heart Failure.2018; 20: 373-381.
Ivabradine effectively lowers the composite incidence of cardiovascular death and HF hospitalization in heart failure (HF) with reduced ejection fraction and sinus rhythm. Bohm M, et al. conducted a study which tackles the two issues that whether the duration of HF symptoms may affect outcomes and whether Ivabradine treatment has any impact on heart rate reduction which is modified by the duration of CHF. In SHIFT, 6505 patients who has chronic HF (left ventricular ejection fraction of ≤35%), sinus rhythm, heart rate of ≥70 b.p.m were treated with guideline-recommended therapies and randomized to placebo or Ivabradine. Outcomes and the impact of Ivabradine treatment in patients with different durations of HF were estimated.
Almost 1416 patients of Ivabradine and 1459 patients of placebo had HF duration of ≥4 weeks and <1.5 years and 836 Ivabradine patients and 806 placebo patients had HF duration of 1.5 years to <4 years, and 989 Ivabradine patients and 999 placebo patients had HF duration of ≥4 years prior to randomization. In the SHIFT population, the assessment of numbers of co-morbidities by HF duration are shown in figure 1. High no of patients showed no or one co-morbidity in the short duration of HF group than in the HF of ≥ 1.5 years. As compared to patients with HF of shorter duration, patients with a HF duration of ≥ 4 years showed three or four co-morbidities frequently. Significantly higher rate of event of primary endpoint was observed in longer duration rather than an intermediate or shorter duration of HF irrespective of treatment with placebo (Figure 2A) or Ivabradine (Figure 2B) (log rank p <0.0001). For cardiovascular death (log rank p <0.0001), identical results were observed. Higher risk was observed with longer disease duration. In the SHIFT population, duration of disease is a risk indicator for cardiovascular outcomes. Irrespective of HF duration, significant reductions in occurrences of the primary composite endpoint and hospitalization for worsening of HF were observed (Figure 3). Nominal reduction was observed in cardiovascular mortality and all-cause mortality. Patients with HF duration of ≥ 4years showed 40% decrease in HF mortality. Effects of Ivabradine were independent of HF duration. Duration of HF predicts results independently of risk indicators for example, higher age, greater severity and more co-morbidities. Reduction of heart rate by Ivabradine leads to improvement in outcomes irrespective of duration of HF. Hence, in future trials according to the chronicity of HF, early HF treatment should be proposed which is critical to represent HF populations.
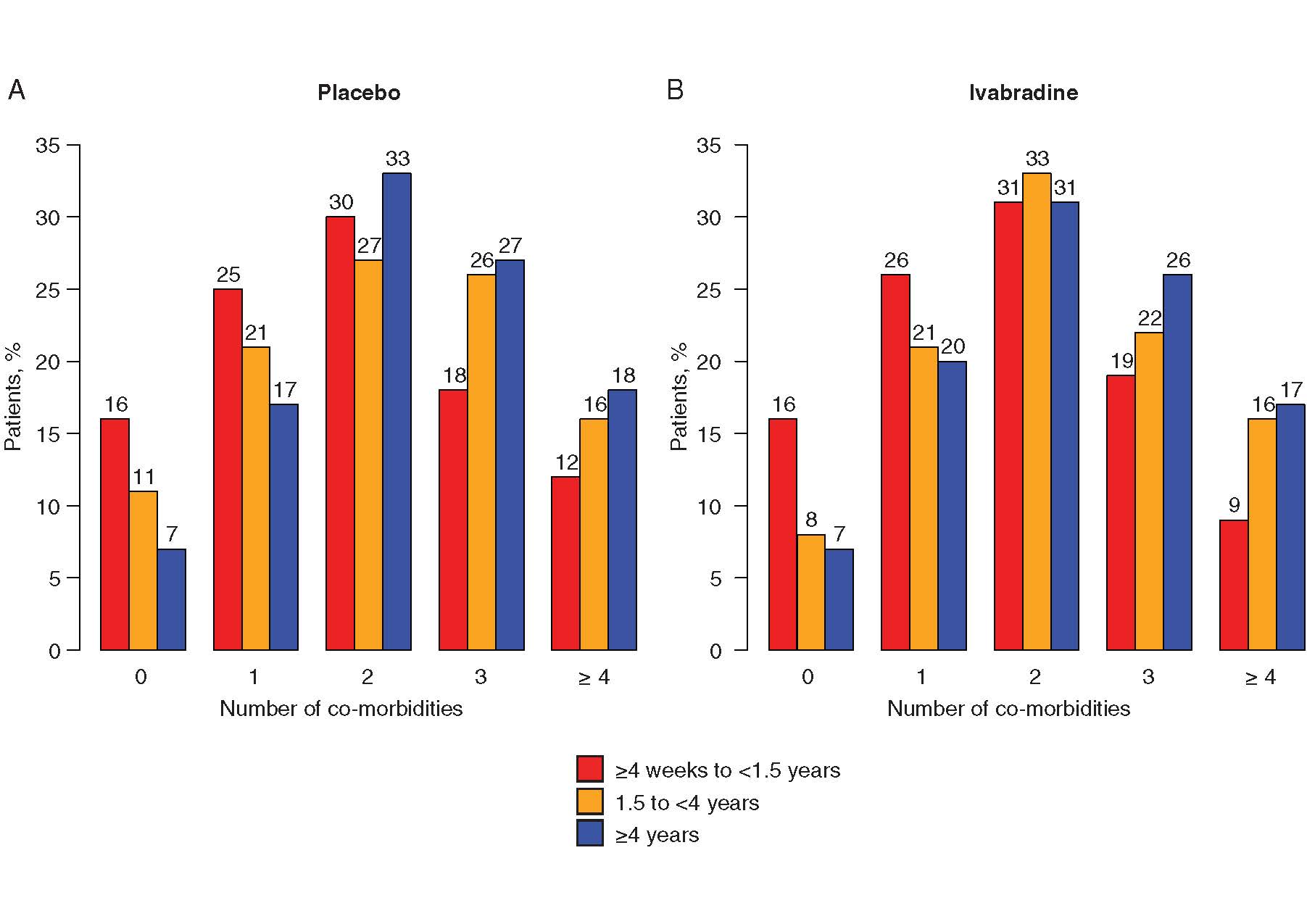
Figure 1. Distribution of numbers of co-morbidities in patients on (A) placebo or(B) Ivabradine according to duration of heart failure (HF) (<1.5 years; 1.5 years to <4years;≥4 years). Percentages were calculated for each class of disease duration. Mean ±standard deviation durations of HF in patients stratified according to number of co-morbidities were: no co-morbidities (n =685): 2.33 ±3.37 years; one co-morbidity (n =1437): 2.95 ±3.83 years; two co-morbidities (n =2008): 3.72 ±4.57 years; three co-morbidities (n =1485): 3.87 ±4.2years;fourormore co-morbidities (n =890): 4.17 ±4.38 years
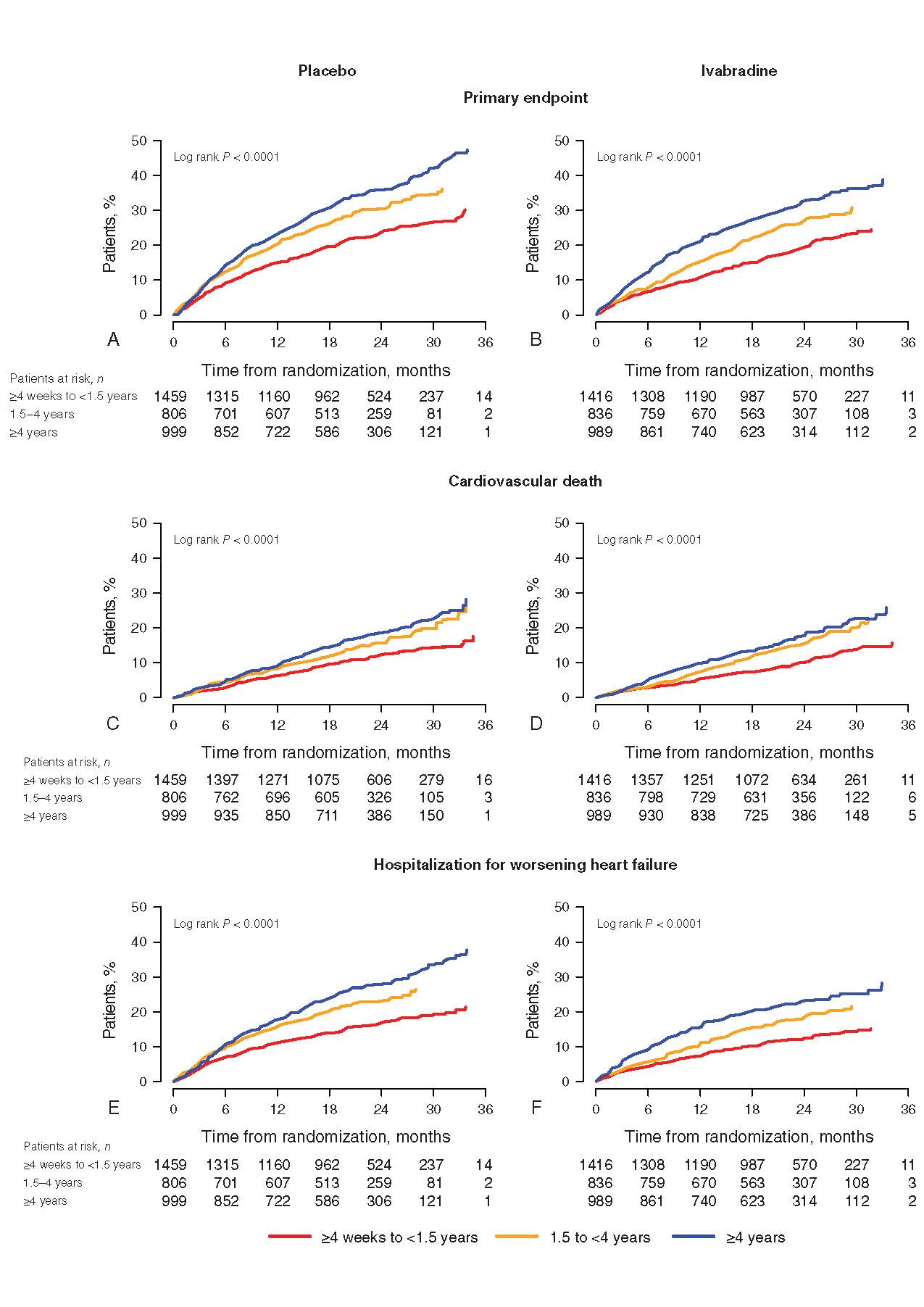
Figure 2. Kaplan–Meier event curves for the (A, B) primary endpoint, (C, D) cardiovascular death and (E, F) hospitalization for worsening heart failure (HF), in patients on (A, C, E) placebo or (B, D, F) Ivabradine according to duration of HF (<1.5 years, 1.5 years to <4years, ≥4years)
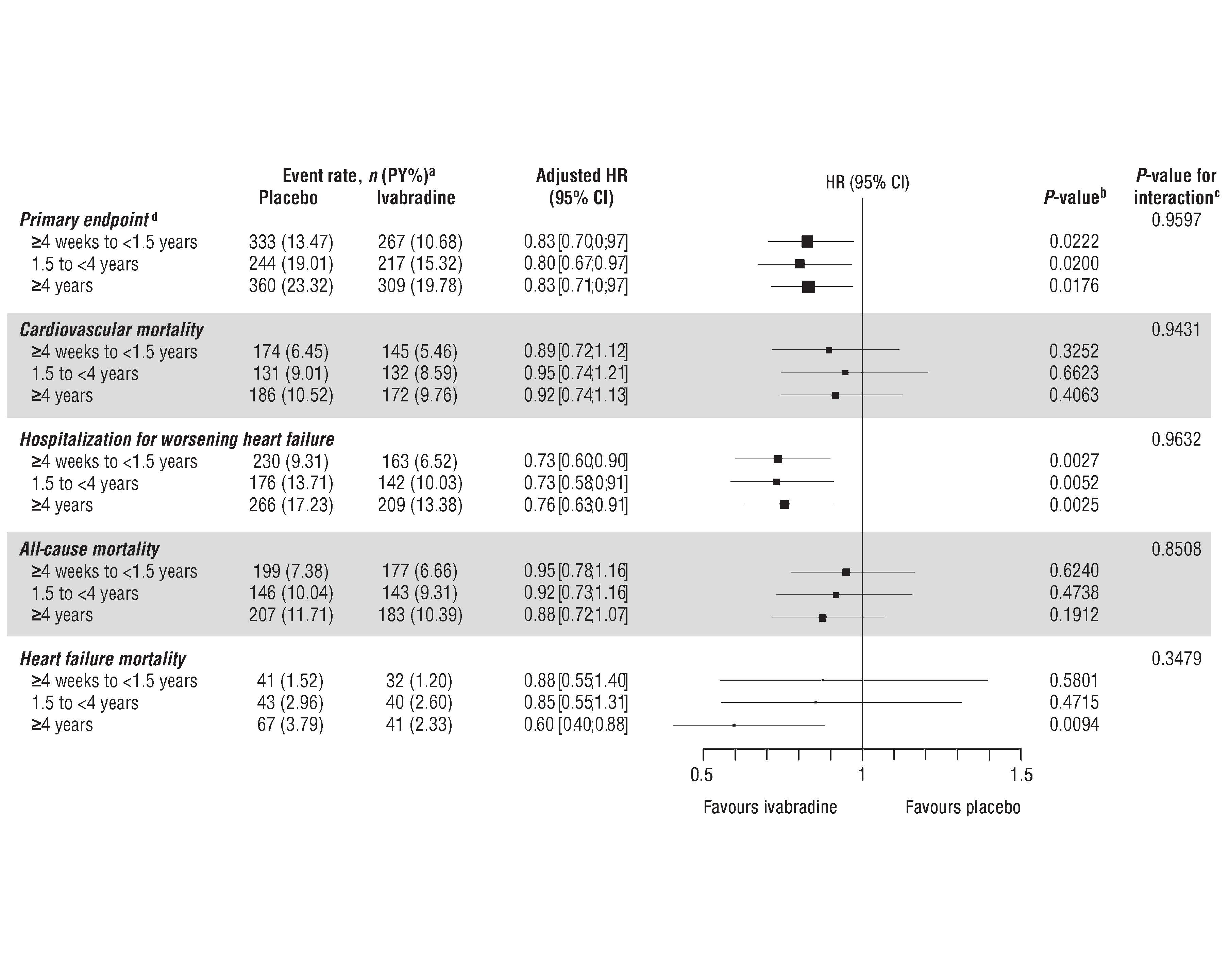
Figure 3. Effects of Ivabradine treatment in different durations of heart failure (HF) indicated by hazard ratios (HR) with 95% confidence intervals (CI) for the primary endpoint, cardiovascular mortality, all-cause mortality and HF mortality according to duration of HF (<1.5 years, 1.5 years to <4years, ≥4years).

