Zachariah D, et al. Int J Cardiol. 2017 Dec 15;249:313-318.
Zachariah D, et al., conducted a LIVE:LIFE, a multi-centre, open-label, prospective observational cohort study to assess the effect of Ivabradine on health-related quality of life (HRQoL) in older patients with CHF.
LIVE:LIFE was implemented in 44 centres across England, Wales, Scotland and Northern Ireland and follow-up was conducted for six months following Ivabradine for treatment of CHF. At 6 months, HRQoL was assessed which was analysed by the Minnesota Living with Heart Failure Questionnaire (MLWHFQ) total score which were included in primary endpoint. At baseline (V1), 2 months (V2) and 6 months (V3), demographic, clinical and HRQoL (MLWHFQ, SF-12) data were compiled. From 44 UK centres, 240 patients were randomised over 14 months. Of 240, Ninety-nine (41%) were female and 28% aged ≥80 years. At both baseline and 6 months (PPS), almost 187 patients completed visits. Ivabradine shows greater reduction in resting heart rate by 13 bpm at 6 months of treatment as compared to 83 bpm with baseline. After 6 months of treatment, 30% of patients improved ≥1 NYHA class, 59% of patient improved global assessment and physician (60%) perspectives. After 6 months, almost 88% of patients were still continuing with Ivabradine.
Ivabradine shows significant improvement by 9 points in MLWHFQ total score as compared with baseline. Ivabradine shows significant improvement in MLWFHQ PDS and MLWHFQ EDS by 4.2 (p < 0.0001, 95% CI −2.8 to −5.7) and 1.5 (p < 0.0001, 95% CI −0.6 to −2.5) after 6 months (Figure 1).
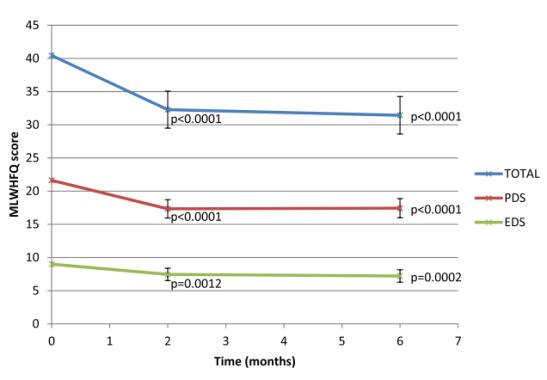
Figure 1: Graph showing mean MLWHFQ score over time for per-protocol set (PPS, n =187). Mean MLWHFQ score over time for PPS (n =187), 95% Confidence Intervals and p-values shown vs. baseline. EDS = Emotional Dimension Score; MLWHFQ = Minnesota Living with Heart Failure Questionnaire; PDS = Physical Dimension Score; PPS = Per-Protocol Set
Greatest improvements were observed in standardised mean MLWHFQ scores were items: 13 (fatigued/low energy), 9 (hobbies difficult) and 12 (short of breath) (Figure 2).
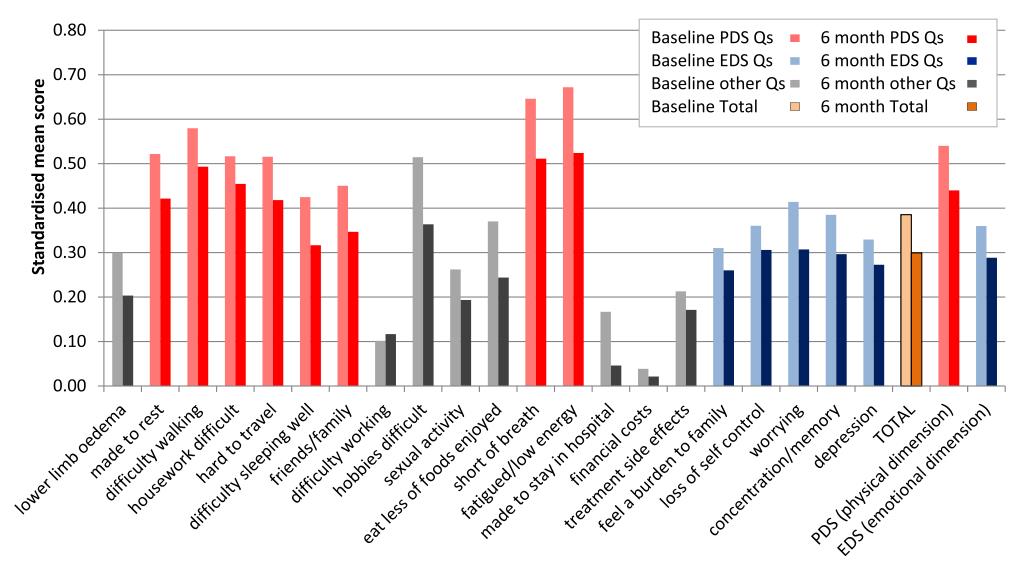
Figure 2: Standardised mean MLWHFQ individual question and summary scores at baseline and 6 months. DS Qs =Emotional Dimension Score Questions; MLWHFQ =Minnesota Living with Heart Failure Questionnaire; PDS Qs = Physical Dimension Score Questions
Thus, it was concluded that 6 months Ivabradine therapy was well tolerated and constantly correlated with improved HRQoL, clinical and functional status in older patients with CHF.

