Metformin for the Management of GDM: Friend or Foe?
F. Dunne
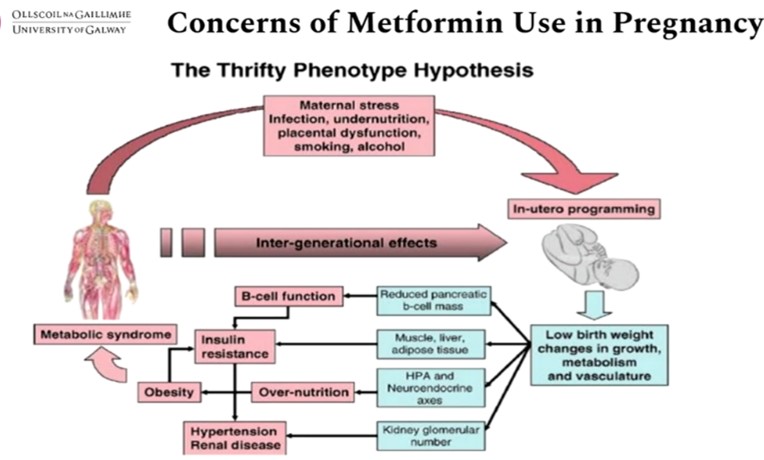
Gestational diabetes mellitus (GDM) is any degree of glucose intolerance with onset or first recognition during pregnancy. GDM represents an underestimated and unrecognised impediment to optimal maternal health & if left untreated it also has severe consequences for the offspring. There are 21 million births globally per year of which 3 million have GDM. The majority of GDM cases are in low- and middle-income countries (LMIC ). Insulin is commonly the treatment of choice after lifestyle changes. Uncertainty exists regarding treatment with metformin.
Dunne elaborated on the role Metformin for the management of GDM in order to understand its role as a friend or foe. The findings were presented at the 59th ESAD Annual meeting held from 2nd -6th October 2023 at Hamburg, Germany. Metformin might be useful in pregnancy because there is significant knowledge and safety data available, it can be administered orally, offers good compliance, is cheap and affordable, has no storage issues & has a global reach including the LMIC.
The NICE 2015 guidelines, offer metformin as first line treatment when lifestyle treatment fails. The ACOG 2017 states that, insulin is the ‘preferred’ approach for GDM for women not sufficiently controlled with diet and exercise. The SMFM 2017 considers metformin to be a “reasonable and safe first-line pharmacologic alternative to insulin”. The ADA 202 states that metformin should not be used as first line treatment.
Early Metformin in Gestational Diabetes, a Randomized Clinical Trial published in JAMA Oct 2023 was a double-blind, placebo-controlled trial conducted in 2 centres in Ireland. Participants were enrolled from June 2017 through September 2022 and followed up until 12 weeks’ postpartum. Participants comprised 510 individuals (535 pregnancies) diagnosed with gestational diabetes based on World Health Organization 2013 criteria. The results indicated that early treatment with metformin was not superior to placebo for the composite primary outcome. Prespecified secondary outcome data support further investigation of metformin in larger clinical trials.
In the Metformin in gestational diabetes: the offspring follow-up (MiG TOFU) study, metformin or insulin for GDM was associated with similar offspring total and abdominal body fat percent and metabolic measures at 7-9 years. Metformin-exposed children were larger at 9 years. Metformin may interact with fetal environmental factors to influence offspring outcomes.
A register-based cohort study from Finland included singleton children born 2004-2016 with maternal pregnancy exposure to metformin or insulin (excluding maternal type 1 diabetes): metformin only (n=3967), insulin only (n=5273) and combination treatment (metformin and insulin; n=889). This study found no increased long-term risk associated with pregnancy exposure to metformin (alone or in combination with insulin), compared with insulin. The increased risk of small for gestational age (SGA) associated with metformin versus insulin suggested caution in pregnancies with at-risk fetal undernutrition.
The Born in Bradford (BiB) study analysed associations between maternal gestational diabetes, metformin or insulin treatment and offspring growth trajectories from birth to 60 months of age. The trial did not show statistical superiority of early metformin over usual care for the composite primary outcome. Metformin had a positive impact on important pre-specified maternal metabolic and neonatal outcomes ie. maternal glycaemic control, maternal weight gain & infant size. Participants assigned to metformin were 25% less likely to require insulin. Metformin was not associated with any increase in maternal or neonatal morbidities.
The author concluded by saying that future trials should be randomized and placebo-controlled. Women should be consented for long-term follow-up of the mother-infant pair. Consideration should be given to bio-banking for interrogation of disease mechanisms. COS and PRO should be used for better data synthesis and meta-analysis.
Tirzepatide Achieves Significant Weight Loss without Adverse Effects on Muscle Composition (SURPASS-3 MRI)
J. Linge
Aging and weight loss commonly result in loss of muscle mass, which is exacerbated in type 2 diabetes (T2D). Tirzepatide is the first dual glucose-dependent insulinotropic polypeptide (GIP)/glucagon-like peptide-1 (GLP-1) receptor agonist receptor co-agonist approved for the treatment of type 2 diabetes in the USA, Europe, and the UAE. Tirzepatide is an acylated peptide engineered to activate the GIP and GLP-1 receptors, key mediators of insulin secretion that are also expressed in regions of the brain that regulate food intake. Tirzepatide, reduced body weight with a beneficial effect on fat distribution pattern in the SURPASS-3 MRI sub study. J. Linge & team examined the effect of TZP on muscle composition and compare observed changes with TZP (5/10/15 mg) or insulin degludec (IDeg) to changes predicted from the longitudinal UK Biobank (UKB) imaging study. The findings were presented at the 59th ESAD Annual meeting held from 2nd -6th October 2023 at Hamburg, Germany.
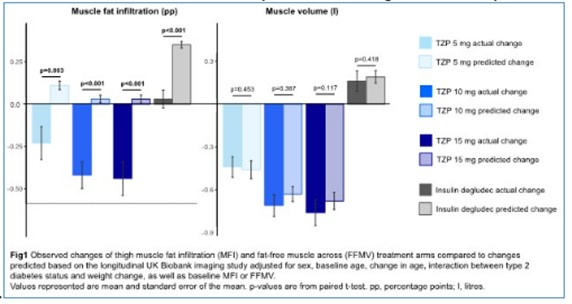
Thigh muscle fat infiltration (MFI), fat-free muscle volume (FFMV), and sex-, height-, and weight-invariant FFMV z-scores were quantified from MRI using AMRA Researcher. Predicted changes for MFI, FFMV, and FFMV z-score in the SURPASS-3 MRI substudy (N=296 participants with T2D inadequately controlled on metformin with/without sodium-glucose co-transporter-2 inhibitors and fatty liver index ≥60) were calculated using data from UKB. All prediction models were fitted to N=2942 UKB participants (scanned ~2.2y apart and included regardless of observed weight change) and adjusted for sex, baseline age, change in age, interaction between T2D status and weight change, as well as baseline value of either MFI, FFMV or FFMV z-score. Paired t-tests were used to compare observed with predicted change.
MFI was significantly reduced across TZP arms (mean [SD] difference -0.23 [0.77]/-0.42 [0.61]/-0.44 [0.81] percentage points [pp] for TZP 5/10/15 mg, respectively), but not for IDeg (+0.03 [0.40] pp); Changes were greater than predicted by UKB (Fig1, left). FFMV was significantly reduced for TZP 5/10/15 mg (-0.44 [0.57]/-0.71 [0.60]/-0.76 [0.74] litres), but not for IDeg (+0.16 [0.54] litres); Changes were overall similar to those predicted by UKB (Fig1, right). FFMV z-score was significantly reduced for TZP 5/10/15 mg (-0.12 [0.33]/-0.23 [0.48]/-0.30 [0.47] SD, but not for IDeg (+0.06 [0.43] SD); Changes were greater than predicted by UKB, but the difference was only significant for TZP 15mg (p=0.004).
In SURPASS-3 MRI, TZP resulted in significantly improved body weight and fat levels without apparent adverse effects on muscle composition, with greater than predicted reduction in MFI and as-predicted reduction in FFMV when compared to changes described by data from the longitudinal UKB imaging study.
Effect of Glucagon-like-peptide 1 Analogues and Sodium-glucose Transport 2 Inhibitors on MACE in an Elderly Population- A Target Trial Emulation in Danish Register Data
V. Kosjerina
In 2019, the estimated number of people older than 65 years living with diabetes was more than 110 million, and by 2045 this figure is expected to reach 276 million. The ageing process and its sequalae of ill health, chronic diseases, and frailty, pose additional clinical challenges and burdens, and complicate the management of type 2 diabetes in older people. Glucagon-like peptide 1 receptor agonists (GLP1-RA) and sodium-glucose cotransporter-2 inhibitors (SGLT2i) have emerged as 2 new classes of antihyperglycemic agents that also reduce significantly the risk of major cardiovascular events, such as the composite of myocardial infarction, stroke, and cardiovascular death. V. Kosjerina & team aimed at finding the effect of glucagon-like-peptide 1 analogues and sodium-glucose transport 2 inhibitors on MACE in an elderly population. The findings were presented at the 59th ESAD Annual meeting held from 2nd -6th October 2023 at Hamburg, Germany.
By linking Danish nationwide registers, a population of elderly individuals with a T2D diagnosis that were new users of either GLP-1, SGLT2i or dipeptidyl peptidase-4 inhibitors (DPP4i) at an age of >=70 years was identified. DPP4i was used as an active comparator, as it has shown a neutral effect on 3-point MACE. Key exclusion criteria were a MACE event (defined as myocardial infarction, stroke, or all-cause mortality) in the 6 months prior to index date or use of any of the study drugs 90 days prior index date. Propensity scores were calculated on baseline covariates using multinomial logistic regression. In an intention-to-treat analysis, we analyzed the incidence of MACE by fitting a Poisson regression model with the index drug as exposure and natural splines logit of the propensity score for GLP1 and SGLT2i and age at follow-up. In a second Poisson model, we allowed for an interaction between age and index drug.
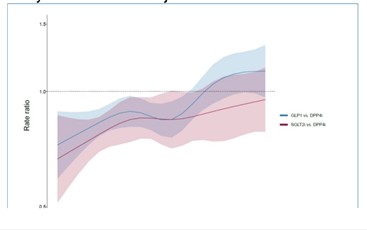
A total of 37,493 individuals met the eligibility criteria (GLP-1 [N= 5,683], SGLT2i (N = 5,000), and DPP4i (N = 26,810]). The age at T2D diagnosis was higher in the DPP4i group (mean [SD]) 69 (8) years compared to GLP-1 63 (8) years and SGLT21 (SD) 65 (8) years. We found a significant reduction in 3-point MACE incidence for GLP-1 with a rate ratio (RR) of 0.90 (95% CI 0.85- 0.95) and for SGLT21 0.83 (95% CI 0.78-0.90), both compared to DPP4i. The figure illustrates the age-specific rate ratios from the interaction model for 3-point MACE, for GLP1 and SGLT2i vs DPP4i.
The author concluded that both GLP-1 and SGLT2i reduced the incidence of MACE compared to DPP4i in this real-world elderly population with T2D. However, there was a reduction of this effect with increasing age at follow-up. Further analysis will include adjustments for relevant covariates during follow-up.
Lower Risk of Severe Hypoglycaemia with SGLT2 inhibitor Compared to DPP4 inhibitor Use in Subjects with Type 2 Diabetes: A Propensity Score-matched Cohort Study
S. Moon
Severe hypoglycemia has been linked to substantial health consequences such as cardiovascular events and mortality. SGLT2 inhibitors reduce hyperglycemia by inhibiting proximal tubular reabsorption of glucose in the kidney; thus, the glucose-lowering effect is independent of insulin secretion. However, SGLT2 inhibitors are unlikely to cause hypoglycemia. Since the glucosuric effect of SGLT2 inhibitors is tied to the filtered load of glucose, they become ineffective once the filtered load reaches ≤80 g/day. SGLT2 inhibitors increase plasma glucagon concentrations and gluconeogenesis in patients with T2D. Thus, the risk of hypoglycemia from SGLT2 inhibitors is low. S. Moon & team investigated the risk of severe hypoglycemia associated with new-use sodium- glucose transport protein 2 (SGLT2) inhibitors compared with dipeptidyl peptidase 4 (DPP4) inhibitors. The findings were presented at the 59th ESAD Annual meeting held from 2nd -6th October 2023 at Hamburg, Germany.
Data from the National Health Insurance Service of Korea was used to identify initiators of SGLT2 or DPP4 inhibitors from 2014 to 2017. By employing a 1:1 propensity score matching technique to control for confounding variables and guarantee a fair comparison, the study included new initiators of SGLT2 inhibitors (n=57,021) and DPP4 inhibitors (n=57,021). The Cox proportional hazards model estimated hazard ratios (HRs) with 95% confidence intervals (CIs) for developing severe hypoglycemia in the matched sample. Exploratory subgroup analyses assessed the consistency of the treatment effects on the primary outcome.
During a 1-year follow-up, the incidence rate of severe hypoglycemia was 1.88 per 1,000 person-years in patients treated with SGLT2 inhibitors and 3.28 per 1,000 person-years in patients treated with DPP4 inhibitors. SGLT2 inhibitors were associated with a significantly lower risk of severe hypoglycemia (HR, 0.57; 95% CI, 0.45-0.73). Subgroup analyses showed that compared with their counterparts, SGLT2 inhibitors significantly decreased the risk of hypoglycemia in high-risk groups for hypoglycemia such as women, patients with peripheral artery disease, and patients on sulfonylurea.
The author concluded that use of SGLT2 inhibitors was associated with a 43% lower risk of severe hypoglycemia than DPP4 inhibitors. SGLT2 inhibitors may be safer in glycemic control than DPP4 inhibitors, especially in subjects at an increased risk of severe hypoglycemia.
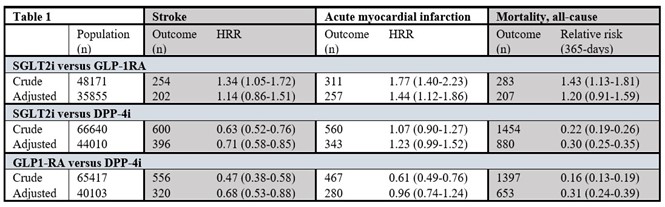
Stroke, Acute Myocardial Infarction and Mortality in Type 2 Diabetes: A Nationwide Comparative Effectiveness Study of GLP-1RA, SGLT2i and DPP-4i Treatment
S. Hastrup
Type 2 diabetes mellitus (DM) is associated with various cardiovascular (CV) complications including hypertension, ischemic heart disease, heart failure, and atherosclerosis. Diabetic patients tend to develop cardiovascular disease at a younger age and at higher incidence than non-diabetic patients. The Framingham Heart Study found that the risks of HF in male and female patients with type 2 diabetes (T2D) are 2.4- and 5-fold higher than that in their counterparts, respectively. In total, 40% of patients with HF have been diagnosed with T2D. Cardiovascular outcome trials (CVOTs) demonstrate that glucagon-like peptide-1 receptor agonists (GLP-1RA) and sodium-glucose cotransporter 2 inhibitors (SGLT2i) reduce the risk of major adverse cardio-vascular events in patients with type 2 diabetes (T2D) and cardiovascular disease (or at high risk), whereas dipeptidyl peptidase-4 inhibitors (DPP-4i) have not shown cardiovascular benefits. Two CVOTs using GLP-1RA also showed significant reduction in non-fatal stroke. Comparative analyses of these newer glucose-lowering medications for prevention of stroke are lacking. The author investigated the risk of stroke (ischemic and hemorrhagic) amongst T2D patients, who were new users of GLP-1RA, SGLT2i and DPP-4i. Further, they investigated the risk of acute myocardial infarction (MI) and all-cause mortality. The findings were presented at the 59th ESAD Annual meeting held from 2nd -6th October 2023 at Hamburg, Germany.
A nationwide population-based cohort study with information from high-quality medical registries with information on hospitalization history, drug use and vital status. Patients with T2D who were new users of GLP-1RA, SGLT2i or DPP-4i in the period 2014-2020 in Denmark were included. Patients with prior stroke were excluded. Cox- and Poisson regressions were used to estimate hazard rate ratios (HRR) and risk ratios (RR), respectively. Inverse probability of treatment weights was applied for the adjusted analyses. Results were adjusted for age, sex, calendar year of initiation, migrant status, co-habitation status, income, education, duration of diabetes, hypertension, atrial fibrillation, Charlson Comorbidity Index (CCI) and diabetes-related chronic complications.
The study included 23,474 new users of GLP-1RA; 24,697 of SGLT2i and 41,943 of DPP4-i. Table 1 shows HRR of stroke and MI; and RR of 365-days all-cause mortality when the groups were compared. New users of GLP1RA and SGLT2i had a lower incidence of stroke when compared to new users of DPP-4i. There was no difference in stroke incidence between the new users of GLP-1RA versus SGLT2i. New users of GLP1-RA showed similar rate of MI when compared to DPP4i. The same was seen when SGLT-2i was compared to DPP4i. GLP-1RA use was associated with a lower risk of MI relative to SGLT2i. Mortality within 365 days was lower in new users of GLP-1RA and SGLT2i compared to new users of DPP-4i.
New users of GLP-1RA and SGLT2i in T2D-patients with and without co-morbid cardiovascular disease were associated with reduced risk of stroke and mortality in comparison with new users of DPP-4i. A wider use of GLP-1RA and SGLT2i may be beneficial in terms of preventing stroke and mortality.
Efficacy and Safety of Real-time Continuous Glucose Monitoring with Remote Monitoring for Diabetes Management in Long-term Care Facilities: A Randomised Clinical Trial
G. Umpierrez
Suboptimal glycemic control persists among a substantial percentage of individuals with type 2 diabetes (T2D). Although use of blood glucose monitoring (BGM) remains common in insulin-treated and noninsulin-treated T2D patients, studies have not consistently shown efficacy in using BGM to change patient behavior or reduce HbA1c in the noninsulin-treated population. The author aimed to evaluate efficacy and safety of real-time continuous glucose monitoring with remote monitoring for diabetes management in long-term care facilities. The findings were presented at the 59th ESAD Annual meeting held from 2nd -6th October 2023 at Hamburg, Germany.
Diabetes is highly prevalent among older adults in subacute and long-term skilled nursing care facilities (LTCF). Management of diabetes in the LTCF residents is challenging due to the high prevalence of comorbidities, altered nutritional intake, which increase the risk of hypoglycaemia. Bedside point-of-care (POC) capillary glucose testing is the standard of care to assess glycaemic control in LTCF; however, it frequently misses asymptomatic hyperglycaemic and hypoglycaemic events.
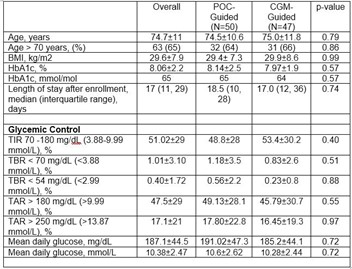
This pilot prospective randomised clinical trial included 100 insulin-treated older adults with type 2 diabetes (T2D) admitted to LTCF. Patients in the standard of care (POC group) wore a blinded Dexcom G6 CGM with treatment adjusted based on POC results. Subjects in the intervention, insulin adjustment was based on daily CGM (Dexcom G6) profile with glucose telemetry system (CGM-GTS group). Treatment adjustment was performed by the LTC medical team, with a target glucose of 140-180 mg/dl (7.77-9.99 mmol/L), and a duration of intervention up to 60 days. Primary endpoint was difference between groups in time in range [TIR, 70-180 mg/dL (3.88-9.99 mmol/L)].
Participants had a mean age of 74.7±11 years, BMI: 29.6±7.9, HbA1c: 8.06±2.2% (65 mmol/mol), with 80% admitted to skilled nursing care/subacute rehab and 20% to long-term care facilities, with a median length of participation of 17 (IQR11, 29) days. There were no significant differences in percentage of time in range (TIR) (53.38% ± 30.16 vs 48.81% ± 28.03, p=0.40), mean daily glucose (185.2 ± 44 vs 191.02 ± 47 mg/dL (10.28 vs 10.61 mmol/L), p=0.72), or the percent of time below range (TBR) (0.83 ± 2.59 vs. 1.18 ± 3.54; P= 0.51 for BG< 70 mg/dL (3.88 mmol/L); 0.23 ± 0.85 vs 0.56 ± 2.24; P=0.88 for BG< 54 mg/dL (2.99 mmol/L) between rt-CGM group and POC-only group.
The author concluded that the results of this pilot RCT indicated that the use of Dexcom G6 rtCGM with remote monitoring is safe and effective in guiding insulin therapy, resulting in a similar improvement in glycaemic control and hypoglycaemia rates compared to POC-guided insulin adjustment in in older subjects with T2D in LTCF.
The Effect of Preprandial versus Postprandial Physical Activity on Glycaemia: Systematic Review and Meta-analysis of Human Intervention Studies
R. Slebe
Poor blood glucose control is a risk factor for the development of type 2 diabetes and cardiovascular disease, even when at subclinical levels. Regular physical activity assists in maintaining blood glucose control, with activity-mediated skeletal muscle glucose uptake able to reduce circulating levels. This is one reason that regular activity is widely promoted to both the general population and subgroups of the population where internal glycaemic regulation may no longer be sufficient. The timing of physical activity (PA) relative to meal intake seems to influence glycaemia, however, no meta-analysis has been performed to directly compare preprandial PA with postprandial PA. One aspect central to blood glucose control is the postprandial response. Repeated bouts of postprandial hyperglycaemia occurring over months and years result in accumulated micro- and macro-vascular damage. Therefore, the TIMED consortium performed this systematic review and meta-analysis to assess the effect of preprandial PA versus postprandial PA on glycaemia in human intervention studies. The findings were presented at the 59th ESAD Annual meeting held from 2nd -6th October 2023 at Hamburg, Germany.
MEDLINE and Embase.com were searched till September 2021 for intervention studies in the general adult population, directly comparing preprandial PA versus postprandial PA on glycaemic measures. Methodological quality was assessed by two independent reviewers and results were meta-analyzed using pooled mean differences in a random-effects analysis or qualitatively described.
Of 23,308 publications screened using AS Review, 25 were included in this review, consisting of 18 acute response studies and 7 RCTs over multiple weeks. Methodological quality was strong (n=10), moderate (n=11) or weak (n=4). In acute response studies, postprandial outcomes followed the expected physiological patterns (e.g. following meal intake), while outcomes measured over 24h showed no significant associations. For the RCTs over multiple weeks, glucose AUC during an OGTT was slightly, but not significantly lower in pre-prandial PA vs postprandial PA (-0.29 mmol/L [95%CI -0.66; 0.08] I2=64.36%). Sensitivity analyses did not significantly change the outcomes.
The conclusion was that this study showed no differences between preprandial PA versus postprandial PA on glycaemia. Longer-term RCTs showed a non-significant potential beneficial effect of preprandial PA. However, more homogeneous high quality long-term RCTs with large populations are needed to strengthen the current evidence.
Investigating Long-term Complications of Gestational Diabetes using Real-world Data
G. Goldet
Gestational diabetes mellitus (GDM) is the most common complication during pregnancy. GDM GDM is defined as glucose intolerance that begins during pregnancy. GDM occurs when the body is no longer able to adapt to its new circumstances, and the endocrine system is unable to produce sufficient insulin. GDM affects approximately 4-10% of pregnancies in the United States annually, depending on the characteristics of the population studied, and the diagnostics utilized. Gestational diabetes mellitus (GDM) is known to be associated with the development of Type 2 Diabetes mellitus (T2DM) and possibly other long-term complications. The author aimed at trying to better understand long-term outcomes for women having suffered GDM in the general population. The findings were presented at the 59th ESAD Annual meeting held from 2nd -6th October 2023 at Hamburg, Germany.
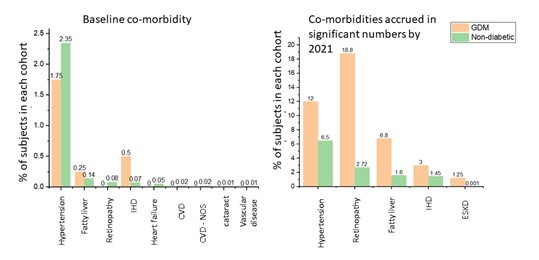
Among 2.3 million people in Northwest London in the DISCOVER-NOW dataset a cohort of 400 subjects were identified coded with GDM in primary care between 2010 and 2011 and followed up through to 2021. The comparator cohort of pregnant women without a coding for any form of diabetes contaied 64, 709 subjects. The team analysed coding for a variety of complications (e.g. Chronic Kidney Disease (CKD), Hypertension, Eye disease, Cerebrovascular disease, Ischaemic heart disease, liver disease, and diabetic foot disease). We also extracted data on baseline demographics.
The authors reported basic demographic data on the two groups including age of first pregnancy, ethnicity, BMI, and smoking status. Though the two groups of women were broadly similar in terms smoking status, BMIs coded for in the GDM group were notably higher. The average age of first pregnancy in the non-diabetic cohort was 30.3 vs. 31.4 in the GDM cohort. They also report co-morbidities accrued in the two groups. The most coded-for comorbidity in the non-diabetic cohort in the follow-up period was hypertension, with 4225 (6.5%) subjects coded for it. In the GDM group, though 48 (12%) did develop hypertension, eye complications were more often coded for, with 75 (18.8%) subjects coded for these (not to mention progression to T2DM which was coded for in 24.5% of GDM subjects vs. 2.5% of non-diabetic subjects).
The author concluded by saying that it has been demonstrated from real-world data, the consequences of GDM over a ten-year period, including development of retinopathy, progression to T2DM and liver disease. A longer follow-up period will provide further details of conditions developing more slowly, such as CKD.

