Dapagliflozin Protects Against Urban Particulate Matter-induced EMT in Tubular Epithelial Cells
Epidemiologic studies have shown that exposure to urban particulate matter (UPM) is associated with chronic kidney disease. Epithelial-to-mesenchymal transition (EMT) is a cellular process during which epithelial cells acquire mesenchymal characteristics and lose their epithelial phenotype. EMT of renal tubular epithelial cells has been considered a mechanism that promotes renal fibrosis. Recent evidence suggested that dapagliflozin, an SGLT2 inhibitor, prevents renal fibrosis in diabetic mice and attenuates EMT of tubular epithelial cells under hyperglycemic conditions. However, whether dapagliflozin affects UPM-induced tubular EMT remains unclear.
Kim J, presented a session at the European Association for the Study of Diabetes (EASD) Annual Meeting held in Hamburg, Germany on 6th October 2023 that evaluated whether UPM is able to induce EMT and dapagliflozin attenuates this process.
HK-2 cells were exposed to nontoxic concentrations of standard reference material for UPM (NIST SRM 1648a). Cells were pretreated with various concentrations of dapagliflozin before exposure to determine whether the EMT process could be inhibited.
Results: HK-2 cells acquired mesenchymal spindle-like morphology after treatment of UPM. In addition, UPM upregulated mesenchymal markers (α-SMA, fibronectin, vimentin) and downregulated epithelial markers (ZO-1, E-cadherin) indicating the development of EMT. Phosphorylation of p38 MAPK significantly increased during EMT while p38 inhibition decreased UPM-induced EMT. Treatment with dapagliflozin attenuated UPM-induced EMT through the inhibition of the p38 MAPK pathway in a dose-dependent manner.
Taken together, these results suggest that UPM directly induces EMT in renal tubular epithelial cells. Our study also provides a rationale to apply SGLT2 inhibitors in non-diabetic kidney diseases such as UPM-related renal fibrosis.
Efficacy of Initial Triple Combination Therapy with Metformin, Dapagliflozin and Saxagliptin in Drug-Naïve Patients with Type 2 Diabetes: A Randomised Controlled Trial
Early combination therapy is recommended in type 2 diabetes (T2D) patients with inadequately controlled hyperglycemia to avoid treatment failure and clinical inertia.
Kin M, presented a session at the European Association for the Study of Diabetes (EASD) Annual Meeting held in Hamburg, Germany on 6th October 2023 that evaluated the efficacy and tolerability of an initial triple combination with metformin, dapagliflozin and saxagliptin compared with conventional stepwise add-on therapy in drug-naïve patients with T2D.
This multicenter, randomized, 104-week, open-label trial was conducted at 9 medical centers in South Korea. Eligible patients were aged ≥18 years, inadequately controlled (HbA1c ≥ 8.0% to <11.0%), drug-naïve patients with recent-onset T2D. Eligible participants were randomly assigned (1:1) to triple combination therapy (TCT, 1000mg of metformin, 10mg of dapagliflozin and 5mg of saxagliptin once daily) or conventional stepwise therapy (CST) group. CST was started with metformin followed by glimepiride, and sitagliptin sequentially. The primary outcome was the proportion of patients who achieved HbA1c <6.5% without hypoglycemia, weight gain ≥5% and discontinuation due to adverse events at week 104.
A total of 105 eligible participants were randomly assigned to either TCT or CST groups. 89.6% of participants completed the study and treatment. The primary outcome was achieved in 42.5% of the TCT group and 17.5% of the CST group (p=0.015). The proportion of achieving HbA1c <6.5% at week 104 was 50.0% of the TCT group and 45.0% of the CST group. Hypoglycemia, weight gain >5%, or discontinuation due to adverse events occurred in 14.9% of the TCT group, and 59.2% of the CST group.
Initial combination therapy with metformin, dapagliflozin and saxagliptin effectively lowered HbA1c with higher tolerability compared with conventional stepwise treatment in recent-onset T2D.
The Gut Hormone GIP Contributes to the Postprandial Gastrointestinal Hyperaemia in Humans
During eating, the blood supply to the digestive organs increases. The gut hormone glucose-dependent insulinotropic polypeptide (GIP) is suggested to play a role in this functional hyperaemia due to vasodilation in the superior mesenteric artery (SMA) accompanied by increased heart rate and reduced blood pressure. The flow-sensitive magnetic resonance imaging (MRI) was used to study.
Gasbjerg L S, presented a session at the European Association for the Study of Diabetes (EASD) Annual Meeting held in Hamburg, Germany on 6th October 2023 that evaluated the effect of exogenous and endogenous GIP on gastrointestinal blood flow.
Ten healthy men (age 21-46 years, BMI 20-26 kg/m2) participated in five randomised MRI scanning experiments (supine position) on separate days: Oral glucose tolerance test (OGTT) + saline infusion, OGTT + GIP receptor antagonist infusion (GIPR-An) (GIP(3-30)NH2 1,000 pmol/kg/min), oral water + GIPR-An, oral water + saline infusion, and oral water + GIP subcutaneous injection (40 nmol). Blood flow was measured repeatedly with phase contrast MRI. Plasma glucose (PG), C-peptide, and heart rate were measured repeatedly.
Results: Blood flow in the SMA and portal vein were stable during water+saline (631±281 and 1,244±35 ml/min, mean±SEM) and water+GIPR-An (746±73 and 1,113±33 ml/min). Baseline divided maximum flow during water+GIP was 149±12% and for OGTT+saline 206±18%. OGTT+GIPR-An resulted in a 47±18% decrease in incremental area under the blood flow curve (iAUC) of SMA compared to OGTT+saline (p = 0.041) (Fig. 1). Also, flow in the portal vein was stimulated by water+GIP and OGTT+saline (135±6.6% and 177±13%), and OGTT+GIPR-An resulted in a 39±13% lower blood flow than OGTT+saline (p = 0.013). Blood flow in the hepatic artery and coeliac trunc, and heart rate were unchanged. PG levels were stable during water+saline, water+GIPR-An, and water+GIP (4.9±0.067, 5.2±0.18 and 4.7±0.072 mmol/l), whereas OGTT+GIPR-An resulted in higher PG levels than OGTT+saline (282±20 vs. 230±25 mmol/l × min, iAUC, p = 0.0051) and lower C-peptide/glucose ratios (3.3±0.73 vs. 5.5±1.1 pmol/mmol × min, iAUC, p = 0.021).
As confirmed by MRI, both endogenous and exogenous GIP increase gastrointestinal blood flow demonstrating that GIP contributes to postprandial hyperaemia. The consequences of GIP receptor targeting treatments remain to be proven.
Clinical Benefit vs Risk of Diabetic Retinopathy Progression with Semaglutide: Watch the Eyes
Rapid reduction in HbA1c can lead to worsening of diabetic retinopathy (DR), as established in pregnancy, insulin initiation and pump therapy.
Jose M, presented a session at the European Association for the Study of Diabetes (EASD) Annual Meeting held in Hamburg, Germany on 6th October 2023 that assessed the changes to DR status with semaglutide use in routine clinical practice for patients with type 2 diabetes mellitus (T2DM).
Patients with T2DM initiated on semaglutide and continuing treatment beyond 6 months were included. Data on DR based on grade of retinopathy (R1-R3), maculopathy, use of laser and anti-VEGF was acquired from the autonomously-run, national retinal screening programme. DR status, prior to initiation of semaglutide and next available DR (at least 3 months beyond initiation) was collected. HbA1c data at point of initiation and follow up (FU) (approximately 3-12 months), were collated.
Results: Of the 126 patients treated with semaglutide, 93 were included in the analysis, based on availability of complete data and continuous use. Mean duration of diabetes was 14.4 years (2-37). At baseline, 56 had no DR and 37 had pre-existent DR. Of the 37, 15 had maculopathy. Seven patients had previous or on-going laser treatment and a further two were on anti-VEGF treatment. Mean HbA1c at initiation was 76 mmol/mol (13-126) and at follow-up was 64 mmol/mol (25-143). The mean duration at which this follow up HbA1c was done was 164 days. DR status progressed in 15 patients during follow up (done at mean 306 days after initiation); nine patients had new development of DR and all had background retinopathy. Six patients had progression of pre-existing DR: one patient developed new maculopathy which needed active surveillance, three with proliferative retinopathy required laser or anti-VEGF treatment. These three cases had active diabetic retinopathy even at initiation of semaglutide warranting ongoing treatment. The mean reduction in HbA1c achieved was significantly greater in patients with progression of DR (n=15, 29.4 ± 21.6 vs 16.7 ± 12.3mmol/mol, p<0.007). The baseline HbA1c was significantly higher in patients with DR progression (89.5 ± 20.0 vs 72.9 ± 17.9 mmol/mol, p<0.002). The diabetes duration (13.4 vs 14.6 years) and proportion of patients on insulin (67% vs 45%) were comparable between the groups. 73 had no change in DR (47 had no DR at baseline or FU, 26 had stable DR) and 5 had improvement in DR during this follow up.
This study once again demonstrates that semaglutide treatment can be associated with significant and rapid reduction in HbA1c, which in turn could increase risk of progression of DR. The risk is higher in patients with higher baseline HbA1c and greater reduction of HbA1c during on-going treatment. This is a retrospective clinical observational analysis and hence inherently involved accepting variations in routine clinical practice such as time between DR screening and HbA1c, disruption to DR screening programme during Covid pandemic and the limitations to monitoring risk factors. However, this study again adds to the evidence that rapid and significant improvement in HbA1c, use of semaglutide being one another instance of this occurring, can increase the risk of development or progression of DR and processes to assess regular HbA1c and more frequent DR screening should be considered.
Durable Improvement in HbA1c in Youth-onset Type 2 Diabetes with and without Background Insulin Therapy: A Post Hoc Analysis of the DINAMO Trial of Empagliflozin and Linagliptin vs Placebo
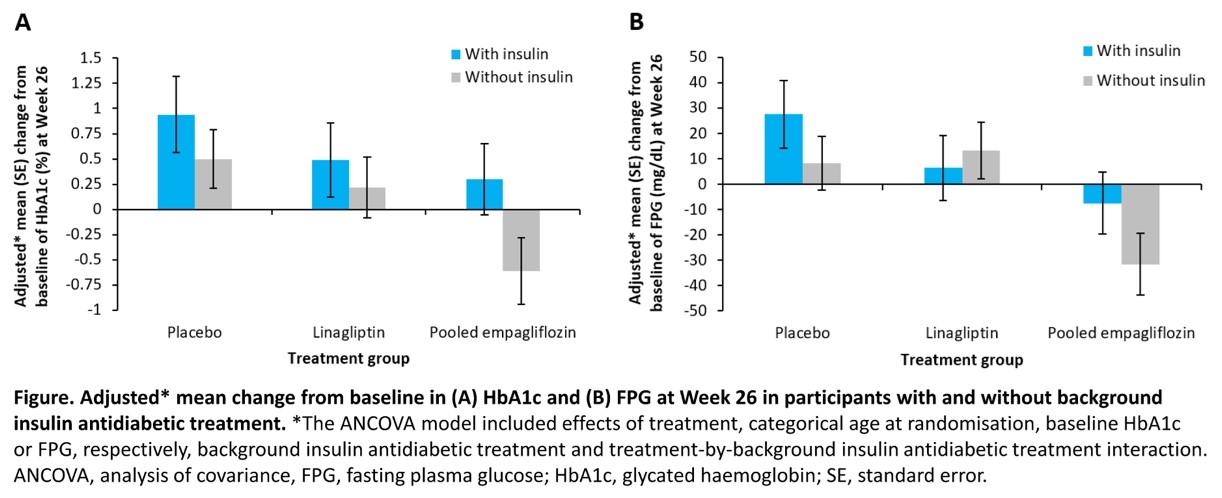
- Willi et al. presented this study at EASD 2023 on 5th October 2023.
The increasing prevalence of childhood overweight and obesity has resulted in a significant rise in type 2 diabetes (T2D) among young individuals. Youth-onset T2D tends to progress more aggressively than in adults, leading to worse blood sugar control and early complications. Currently, metformin is the only approved oral treatment for youth with T2D, and all other options involve injections. Therefore, there is a pressing need for additional oral therapies.
The DINAMO trial aimed to assess the effectiveness and safety of empagliflozin and linagliptin compared to a placebo in young people with T2D. This specific analysis focused on how the presence of background insulin therapy affected blood sugar outcomes over a 52-week period.
In the trial, 157 participants aged 10-17 were randomly assigned to receive empagliflozin 10 mg, linagliptin 5 mg, or a placebo. Participants with background treatment involving metformin and/or insulin were included. For those in the empagliflozin group who did not achieve the target glycated hemoglobin (HbA1c) level of <7.0% by Week 12, further randomization (1:1) to empagliflozin 10 or 25 mg was conducted, and their outcomes were combined.
Results showed that empagliflozin led to a significant reduction in HbA1c and fasting plasma glucose (FPG) from baseline to Week 26 in participants not receiving insulin as background therapy. The improvements were sustained up to Week 52.
Empagliflozin could offer a valuable new treatment option for youth with T2D, potentially avoiding the need for injectable therapies.
Comparative Effectiveness of GLP-1 Receptor Agonists vs DPP-4 inhibitors in Patients with Type 2 Diabetes and Moderate/Severe Chronic Kidney Disease
K R. Tuttle et al. presented this study at EASD 2023 on 5th October 2023.
Patients with type 2 diabetes (T2D) who also have chronic kidney disease (CKD) often face limited treatment options due to their impaired kidney function, which can result in the accumulation of medications and their byproducts. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have demonstrated effectiveness in managing blood sugar levels and promoting weight loss in T2D patients with reduced kidney function. However, there is a need to compare the real-world effectiveness of GLP-1RAs with dipeptidyl peptidase-4 inhibitors (DPP-4is), another commonly used class of diabetes medications, in this population. This study aims to assess how GLP-1RAs compare to DPP-4is in terms of glycemic control and body weight reduction in T2D patients with moderate or severe CKD.
In this study, patients aged 18 years or older with T2D and moderate or severe CKD, identified from the Clinical Practice Research Datalink Aurum database, initiated either a GLP-1RA or DPP-4i between 2007 and 2022. Patients were matched in a 1:1 ratio based on propensity scores across treatment groups. Changes in HbA1c levels and body weight over a 6-month period were assessed using a mixed model with repeated measures, including baseline values and all available follow-up measurements.
The analysis included 1,650 GLP-1RA initiators and 1,650 DPP-4i initiators, with similar characteristics at baseline. After 6 months, GLP-1RA treatment led to a significantly greater reduction in HbA1c compared to DPP-4i treatment. Among patients with baseline HbA1c levels ≥7%, a similar proportion achieved HbA1c levels <7.0% with both GLP-1RAs and DPP-4is. Additionally, GLP-1RAs were associated with a significantly larger reduction in body weight compared to DPP-4is, and a greater percentage of GLP-1RA-treated patients achieved a body weight reduction of more than 5%.
In patients with T2D and moderate or severe CKD, GLP-1RAs appear to be more effective in reducing HbA1c levels and promoting weight loss compared to DPP-4is. Therefore, GLP-1RAs may be a preferable choice for managing hyperglycemia and achieving weight-related outcomes in this patient population.
Remission of Type 2 Diabetes with the Oral Combo Semaglutide + Dapagliflozi
- E. Lunati et al. presented this study at EASD 2023 on 5th October 2023.
Combining a glucagon-like peptide-1 receptor agonist (GLP-1RA) with a sodium-glucose co-transporter-2 inhibitor (SGLT2i) has shown promising results in improving glycometabolic outcomes. However, the real-world effectiveness of this combination therapy remains understudied. In this prospective multicenter study, we aimed to assess the efficacy of the oral Semaglutide/Dapagliflozin combination therapy compared to Dapagliflozin alone as an add-on therapy for patients with type 2 diabetes (T2D) in a real-world setting.
We enrolled a total of 523 patients with T2D who initiated oral Semaglutide/Dapagliflozin combination therapy and compared their clinical outcomes with 415 patients receiving Dapagliflozin alone over a 6-month follow-up period. Baseline characteristics, including comorbidities and cardiovascular events, were documented.
The patients in the combination therapy group (mean age 62.4±7.5 years, F/M 250/273) received oral Semaglutide (mean dosage: 12.7±2.7 mg/day) and Dapagliflozin (10±0.0 mg/day) as add-on therapy to Metformin in 38.6% and insulin treatment in 2.1% of subjects. In contrast, the Dapagliflozin alone group (mean age 67.1±10.1 years, F/M 155/260) received Dapagliflozin (mean dosage 10.0±0.0 mg/day) as an add-on to Metformin in 76.8%, insulin treatment in 13%, and other antidiabetic drugs in 6%. After 6 months, the combination therapy group exhibited significantly greater reductions in HbA1c (-1.1±0.7 vs. -0.5±0.6%, P<0.001), BMI (-1.5±1.2 vs. -0.9±0.9 Kg/m², P<0.001), and fasting plasma glucose (-32.5±29.2 vs. -15.2±29.4 mg/dL). Additionally, the combination therapy enabled a higher percentage of patients to achieve target HbA1c levels (≤7.0% in 77.8%, ≤6.5% in 51%, and ≤6.0% in 13.5%) compared to the Dapagliflozin group (48.1%, 24.8%, and 7.9%, respectively) without increasing the risk of severe hypoglycemia. Renal function remained stable, while albumin-to-creatinine ratio (ACR) values decreased in both groups, indicating an improved renal risk profile.
The combination therapy of GLP-1RA and SGLT2i (oral Semaglutide/Dapagliflozin) leads to significant improvements in glycometabolic outcomes, including diabetes remission in over 50% of patients, without an increased risk of hypoglycemia. Moreover, this therapy benefits renal function, offering a comprehensive approach to managing T2D.

