Seferovic PM. Eur J Heart Fail. 2019 Dec 9.
New glucose-lowering medications i.e. DPP-4 inhibitors, GLP-1 RA, and SGLT-2 inhibitors impacts glycaemic control and causes CV risk decrease in T2DM. At present, the exact procedure(s) of favourable CV impacts of these medications are unclear in humans and are under evaluation in particular mechanistic studies. Hence, Seferovic PM et al., conducted a study to outline clinical trial data on the role and safety of these new evidence-based therapies for the therapy of T2DM in patients with HF.
The FDA and the EMA, from 2008 and 2012, respectively, have emphasized that CV outcome trials (CVOTs) analysing novel glucose-lowering medications are designed to assess CV safety. The primary outcome was a composite of the three major adverse CV occurrences (3-point MACE) comprising CV death, non-fatal myocardial infarction (MI) and non-fatal stroke in the majority of CVOTs. Hospitalisation for unstable angina (4-point MACE) was also incorporated in two trials, and two co-primary outcomes (the 3-point MACE and a composite of CV mortality and HF hospitalisation) was shown in one trial. The CVOTs with DPP-4 inhibitors (saxagliptin, alogliptin, sitagliptin, and linagliptin) have indicated non-inferiority in respect to primary 3-point MACE as compared to placebo, but they do not exhibit advantage. Either a higher risk of HF in patients with established CV disorder, or a greater HF risk correlated with saxagliptin, but not with other DPP-4 inhibitors has been indicated in several meta-analyses of these trials. A recently presented CAROLINA trial showed no difference in the 3-point MACE effect and no rise in the risk of HF hospitalisation (p =0.176) among linagliptin and an active comparator, glimepiride, but glimepiride treated patients had more hypoglycaemia as compared with those receiving linagliptin. The CV safety profile of the subcutaneous GLP-1 RA class of agents (lixisenatide, liraglutide, semaglutide, exenatide, albiglutide and dulaglutide) was evaluated by six CVOTs and the first orally active form of the GLP-1 RA, oral semaglutide was assessed by one trial. Although to a varying degree for individual drugs, these medications can decrease the rate of 3-point MACE recommended by a meta-analysis of the four trials with a GLP-1 RA. Thus GLP-1 RA have exhibited a neutral impact on the risk of HF hospitalisation, with a beneficial movement seen with liraglutide, albiglutide and oral semaglutide. Liraglutide had a neutral impact on LVEF in patients with chronic stable HFrEF (with or without T2DM), but led to enhanced heart rate and more adverse CV occurrences as compared with placebo in the recent LIVE study. Further analysis should merit for the recommended safety signal with some of the GLP-1 RA in patients with HFrEF. In the proximal renal tubule, SGLT-2 inhibitors (empagliflozin, canagliflozin, dapagliflozin, ertugliflozin) have a unique glucose-lowering impact via preventing glucose reabsorption. Especially, SGLT-2 inhibitors are the first class of glucose-lowering medications that have indicated a positive impact on decrease in risk for HF hospitalisation (Figure 1).
DPP-4 inhibitors are well tolerated, weight-neutral and correlated with a low risk of hypoglycaemia in the general population of T2DM patients. GLP-1 RA indicated a neutral impact on the risk of HF, and liraglutide, albiglutide and oral semaglutide showed lower risk. Correspondingly, SGLT-2 inhibitors have been suggested as an add-on therapy in patients who have not gained sufficient glucose control with metformin (or in whom metformin is contraindicated/not tolerated). SGLT-2 inhibitors are correlated with a low risk of hypoglycaemia and in order to gain optimum glucose control, it can be securely and efficaciously combined with other glucose-lowering drugs.
Thus, it was concluded that this class of medications has a beneficial safety profile with low risk of hypoglycaemia and advantageous impact on weight control, while serious adverse occurrences (e.g. ketoacidosis, bone fracture or limb amputations) arised unusually and with appropriate patient selection and monitoring, it could be avoided.
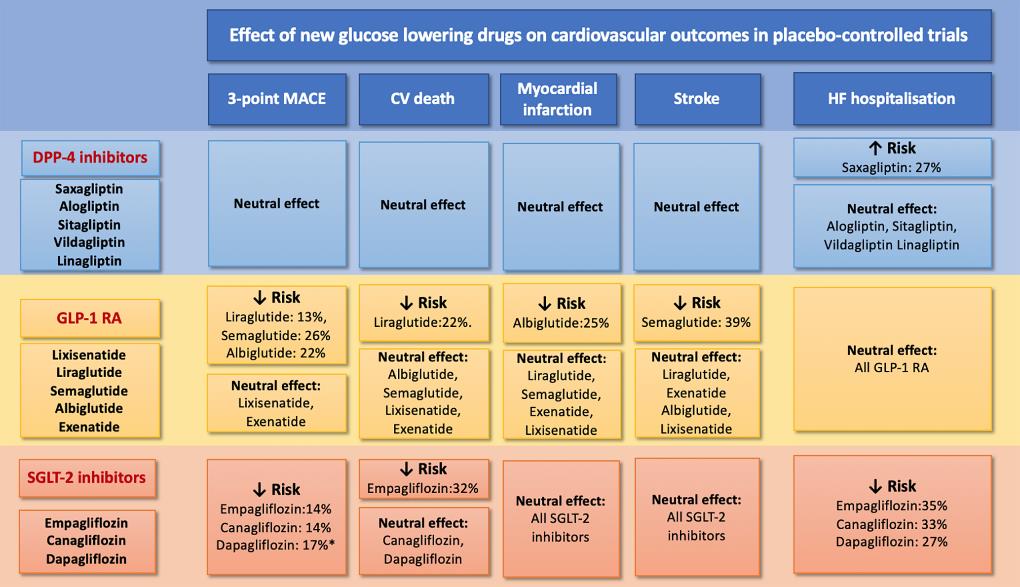
Figure 1: Summary of clinical trial results with new glucose-lowering medications in patients with type 2 diabetes mellitus
DPP4: Dipeptidyl peptidase-4; GLP-1 RA: glucagon like peptide-1 receptor agonists; SGLT2: sodium–glucose co-transporter type 2; CV: cardiovascular; FDA: Food and Drug Administration; EMA: European Medicines Agency; CAROLINA: Cardiovascular Outcome Study of Linagliptin versus Glimepiride in Patients with Type 2 Diabetes; LIVE study: Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes

