Boster J, et al. Cureus 11(9): e5708.
Boster J, et al., conducted a study to estimate the effect of adoption of coronary artery disease-reporting and data system (CAD-RADS) on downstream testing, referral for cardiology consultation, and direct costs in a single-center closed referral system.
For almost 1,796 patients undergoing coronary CT angiography (CCTA) before and after the adoption of the CAD-RADS reporting template at a single-center closed referral hospital system, downstream testing, cardiology referral, and cost were reviewed. Earlier to local CAD-RADS adoption, CCTAs following 12-month duration were included in the non-standardized reporting (NSR) cohort. Following CAD-RADS adoption, CCTAs carried out in the 12 months were included in the CAD-RADS cohort. By usage of the Center for Medicare & Medicaid Services (CMS) outpatient prospective payment system (OPPS) final rule for 2018, cost analysis depends on direct invasive and non-invasive testing.
During the CAD-RADS adoption period, the CAD-RADS recording device was applied in 83.7% of CCTAs. Between the CAD-RADS cohort and NSR cohort groups, baseline cardiovascular risk factors were balanced. No difference in referrals for additional downstream testing was observed between the CAD-RADS and NSR cohorts (10.7% vs 10.8%, p = 0.939) (Figure 1). As compared to NSR, among patients with non-obstructive CAD, 5.1% reduction was observed in referral for downstream testing in CAD-RADS 1 & 2 patients vs. 14.4%, p < 0.001 (Figure 1). This was counteracted by more non-diagnostic scans in the CAD-RADS cohort (9.7% vs 4.2%, p < 0.001), leading to rise in downstream testing (28.8% vs. 11.4%, p = 0.038) (Figure 1).
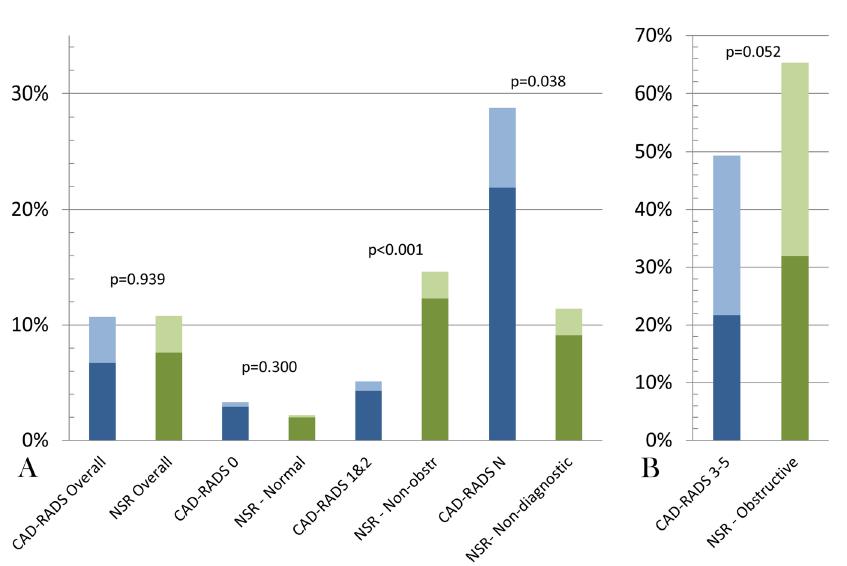
Figure 1: Downstream testing rates before and after the adoption of the CAD-RADS standardized reporting template Downstream testing in the CAD-RADS (blue) compared with NSR (green) cohorts. The dark color represents functional testing and the lighter color represents coronary anatomy testing. Panel A: Graphical representation of CAD-RADS and NSR cohorts overall and with nonobstructive/ non-diagnostic scans; Panel B: Graphical representation of CAD-RADS and NSR cohorts with obstructive coronary artery disease CAD-RADS, coronary artery disease-reporting & data system; NSR, non-standardized reporting; Non-obstr, non-obstructive; N, non-diagnostic.
Overall, no difference was observed between the groups in cardiology referral rates by primary care providers (PCPs) (12.2% vs 15.8%, p = 0.197). Among patients with non-obstructive CAD, cardiology referral rates were increased by 20.5% in in the NSR cohort as compared to 8.6% in CAD-RADS 1 & 2 patients, p = 0.021. Overall, in both groups, referrals for invasive coronary angiography were low (3.5% vs. 3.2%, p = 0.726).
When CAD-RADS or NSR templates were utilized, median downstream total testing costs were similar ($1248; $526-$7750 vs $1248; $825-$7750, p = 0.554) (Figure 2).
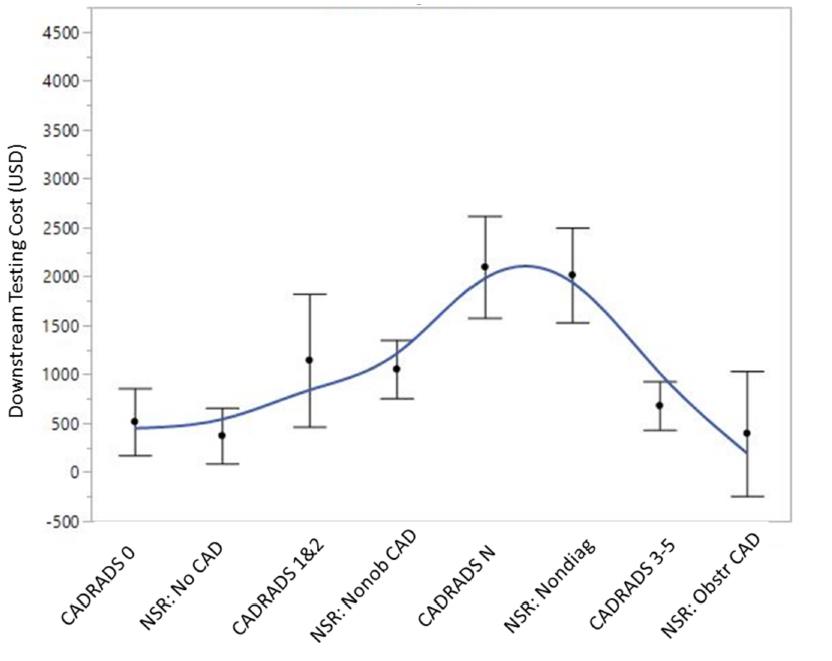
Figure 2: Comparison of median downstream testing costs between the CAD-RADS and non-standardized reporting cohorts Spline curve with error bars depicting the median downstream testing costs based on per-patient maximum coronary stenosis between the CAD-RADS and NSR cohorts. p = NS for all between-group comparisons. CAD-RADS, coronary artery disease-reporting & data system; Nonob CAD, nonobstructive coronary artery disease; Nondiag, non-diagnostic; Obstr CAD, obstructive coronary artery disease; NSR, non-standardized reporting; USD, United States Dollar.
Among non-obstructive CAD (CAD-RADS 1 & 2) patients, association was observed between adoption of the CAD-RADS reporting template with a reduction in downstream testing and cardiology referral rates. Hence, CAD-RADS may have impact on downstream testing in patients in whom additional testing can usually be delayed.

