In clinical trials, once-weekly subcutaneous semaglutide reduced the risk of HF outcomes compared with placebo in individuals with HF (including those with preserved ejection fraction (HFpEF), obesity or chronic kidney disease). Data to benchmark results, in patients with cardiometabolic HFpEF semaglutide and tirzepatide showed >40% risk reduction for the composite of hospitalisation for HF or all-cause mortality compared with a placebo proxy. Rodica Pop-Busui aimed to assess the effects of oral semaglutide on heart failure outcomes in SOUL. The findings were presented at the EASD Annual th st Meeting 2025, held from 15 -19 September 2025 in Vienna, Austria.
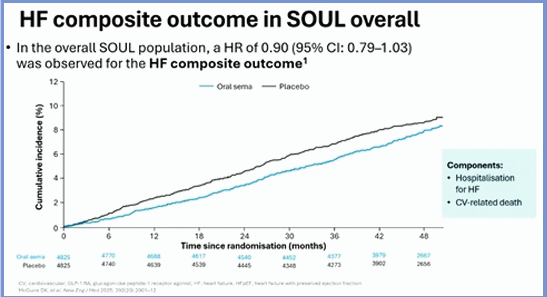
In the trial, participants who were 50 years of age or older, had type 2 diabetes with a glycated hemoglobin level of 6.5 to 10.0%, and had known atherosclerotic cardiovascular disease, chronic kidney disease, or both received either once-daily oral semaglutide (maximal dose, 14 mg) or placebo, in addition to standard care. This prespecified SOUL post hoc analysis aimed to evaluate the effect of oral semaglutide on HF events in participants with or without HF at randomisation & assess the centrally adjudicated HF composite outcome time to first occurrence of HF events (HF hospitalisation or urgent HF visit) & CV death.
Individuals with HF in NYHA functional class IV were not eligible. The main outcomes were time to the first occurrence of HF composite outcome by HF status at baseline. For participants with HF at baseline, the incidence rate of HF events was lower with oral semaglutide (1.75%) compared to placebo (2.23%), with a hazard ratio (HR) of 0.78 (95% CI: 0.57–1.07). The interaction p-value was 0.37, indicating no significant difference in effect between those with or without baseline HF. For participants without HF at baseline, the incidence rates of HF events were similar between semaglutide & placebo groups.
For CV death in those with HF at baseline, oral semaglutide had an incidence rate of 2.65% vs. 3.09% for placebo. Overall, oral semaglutide shows a trend toward reduction of HF events and CV death in patients with HF at baseline. The safety profile in participants with HF at baseline indicates that serious adverse events were reported in 53.8% of the oral semaglutide group and 57.1% of the placebo group. No differences in serious adverse events were observed between oral semaglutide and placebo in participants with or without HF at baseline, despite high use of concomitant medication.
The author concluded by saying that in SOUL trial, oral semaglutide reduced the risk of HF events or CV death in those with a history of HF versus placebo. Benefits were attributed mostly to those with HFpEF. There was no indication of compromised safety in individuals with HFrEF. Benefits were observed despite high baseline use of SGLT2i or MRA, without increasing the risk of SAEs. These data support the initiation of oral semaglutide in people with T2D and HF to reduce HF events.

