
Heart failure (HF) represents a prevalent and prognostically important complication in individuals with type 2 diabetes (T2D), contributing substantially to morbidity and mortality. Although glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have shown cardiovascular benefit in T2D, the impact of the once-daily oral semaglutide formulation on HF outcomes has not been fully characterized. The SOUL trial was a multinational, randomized, double-blind, placebo-controlled cardiovascular outcomes study in adults with T2D and atherosclerotic cardiovascular disease (ASCVD) and/or chronic kidney disease (CKD), originally designed to assess major adverse cardiovascular events (MACE). This secondary analysis evaluates the effect of oral semaglutide on HF outcomes according to HF status at baseline.
The phase 3 CORALreef Lipids trial, a multinational, double-blind, randomized, placebo-controlled study published in the New England Journal of Medicine on February 4, 2026, assessed the efficacy and safety of enlicitide decanoate, an investigational oral macrocyclic peptide inhibitor of proprotein convertase subtilisin/kexin type 9 (PCSK9). Enlicitide targets PCSK9 to increase hepatic LDL receptor availability and reduce circulating LDL cholesterol (LDL-C), offering a convenient oral alternative to injectable monoclonal antibodies.
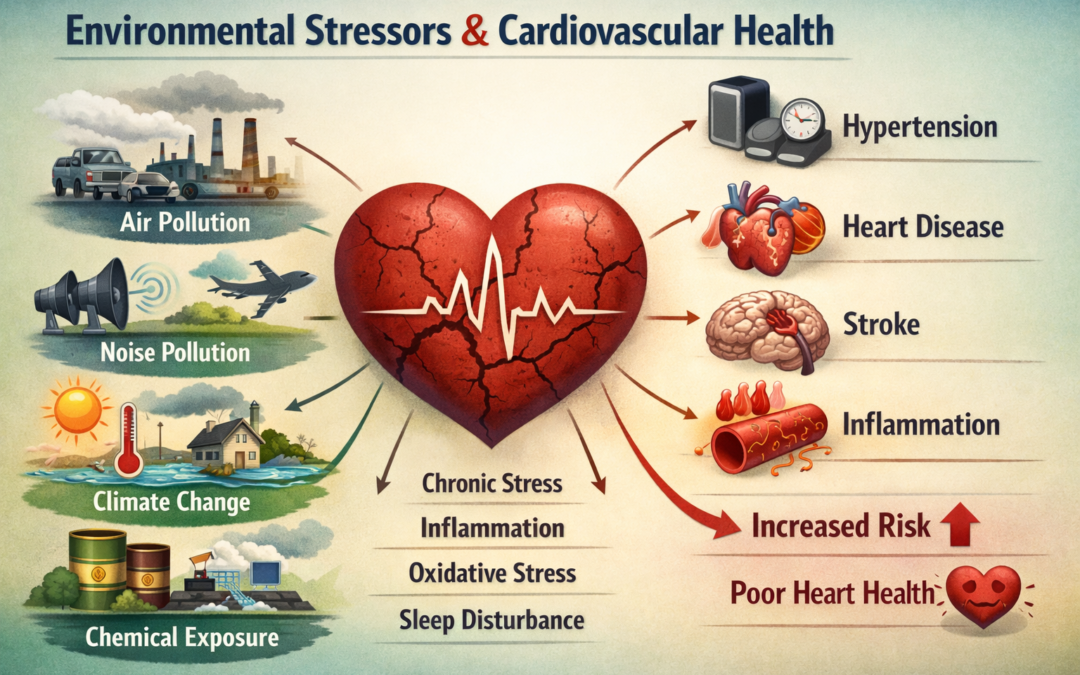
Non-communicable diseases (NCDs) account for 70% of global mortality, claiming over 38 million lives each year, with cardiovascular disease (CVD) as the leading contributor. While conventional risk factors like smoking, hypertension, and poor diet remain critical, emerging evidence highlights the escalating role of ubiquitous environmental risk factors (ERFs) in driving the rise of NCDs, particularly CVD. These interconnected anthropogenic exposures—air pollution, noise and light pollution, chemical and plastic contamination, water and soil pollution, and climate-related hazards—exert cumulative and compounding effects on cardiovascular health. They operate through shared pathophysiological mechanisms, including oxidative stress, systemic inflammation, autonomic nervous system imbalance, and endothelial dysfunction, amplifying overall risk beyond traditional factors.
Tirzepatide, a dual agonist of the glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptors, has demonstrated superior glycemic control and greater weight reduction compared with GLP-1 receptor agonists alone in patients with type 2 diabetes. Dulaglutide, a GLP-1 receptor agonist, has established cardiovascular benefits in this population. However, the cardiovascular effects of tirzepatide relative to dulaglutide remain unknown.
Chagas disease, caused by Trypanosoma cruzi, is a leading cause of non-ischemic cardiomyopathy in Latin America, affecting 6–7 million people. Despite clear benefit of sacubitril/valsartan over enalapril in HFrEF of other etiologies (PARADIGM-HF trial), its efficacy in Chagas cardiomyopathy remained unknown due to distinct pathophysiology involving chronic inflammation, fibrosis, and microvascular dysfunction.

Tirzepatide, a dual GLP-1/GIP receptor agonist, induces substantial weight loss and cardiometabolic improvements in adults with obesity. However, the SURMOUNT-4 trial demonstrated that most participants regain weight upon discontinuation, raising questions about the durability of associated health benefits. The objective is to evaluate changes in cardiometabolic parameters stratified by the extent of weight regain following tirzepatide withdrawal in the SURMOUNT-4 trial.







