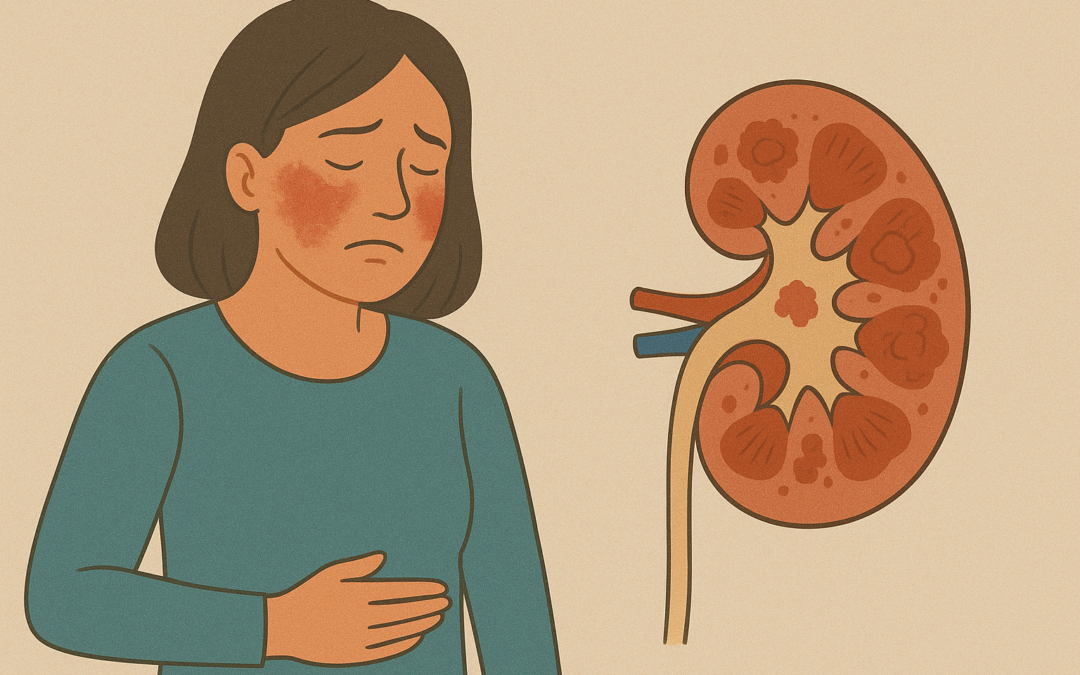
On October 20, 2025, the U.S. Food and Drug Administration (FDA) granted approval to Roche’s Gazyva/Gazyvaro (obinutuzumab) for the treatment of lupus nephritis (LN), a severe complication of systemic lupus erythematosus (SLE) that affects the kidneys. Lupus nephritis is characterized by inflammation that can lead to kidney damage, impaired function, and, in severe cases, kidney failure.

Teva Pharmaceuticals and Medincell announced on October 10, 2025, the U.S. FDA’s approval of an expanded indication for UZEDY® (risperidone) extended-release injectable suspension, now authorized for subcutaneous use as monotherapy or adjunctive therapy to lithium or valproate for maintenance treatment of bipolar I disorder (BD-I) in adults. This marks UZEDY’s second major indication following its 2023 approval for schizophrenia treatment in adults, where it is administered every one or two months.
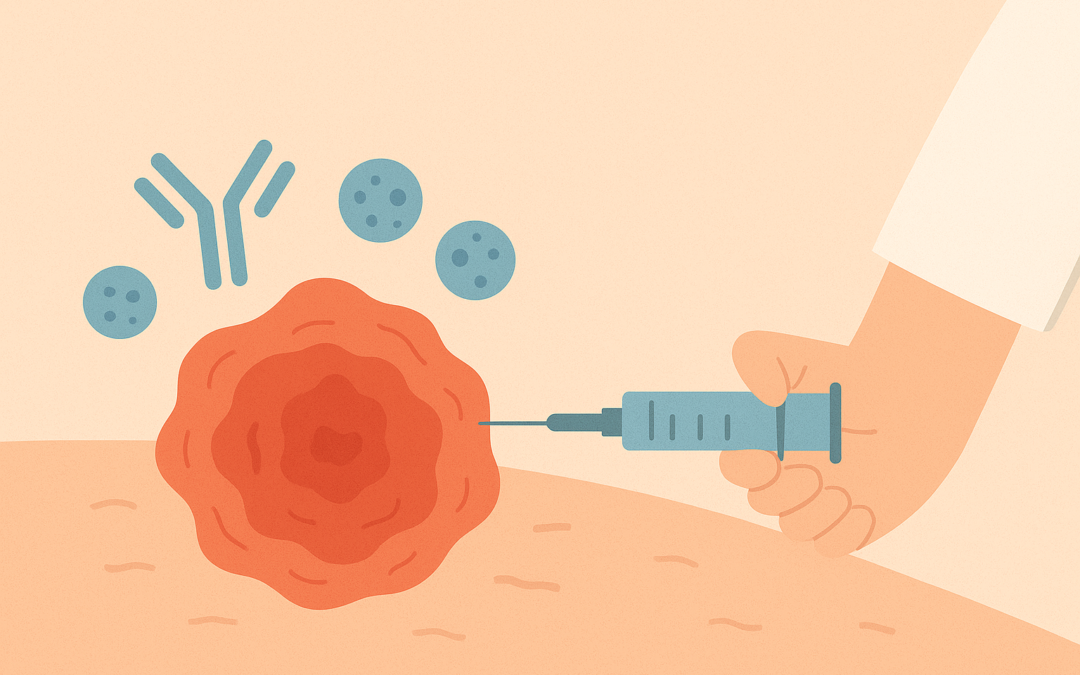
On October 8, 2025, Regeneron Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) approved Libtayo (cemiplimab-rwlc), a PD-1 inhibitor, as the first and only immunotherapy for adjuvant treatment of adult patients with cutaneous squamous cell carcinoma (CSCC) at high risk of recurrence following surgery and radiation. This approval, evaluated under Priority Review, is grounded in data from the pivotal Phase 3 C-POST trial, published in the New England Journal of Medicine and presented at ASCO 2025.
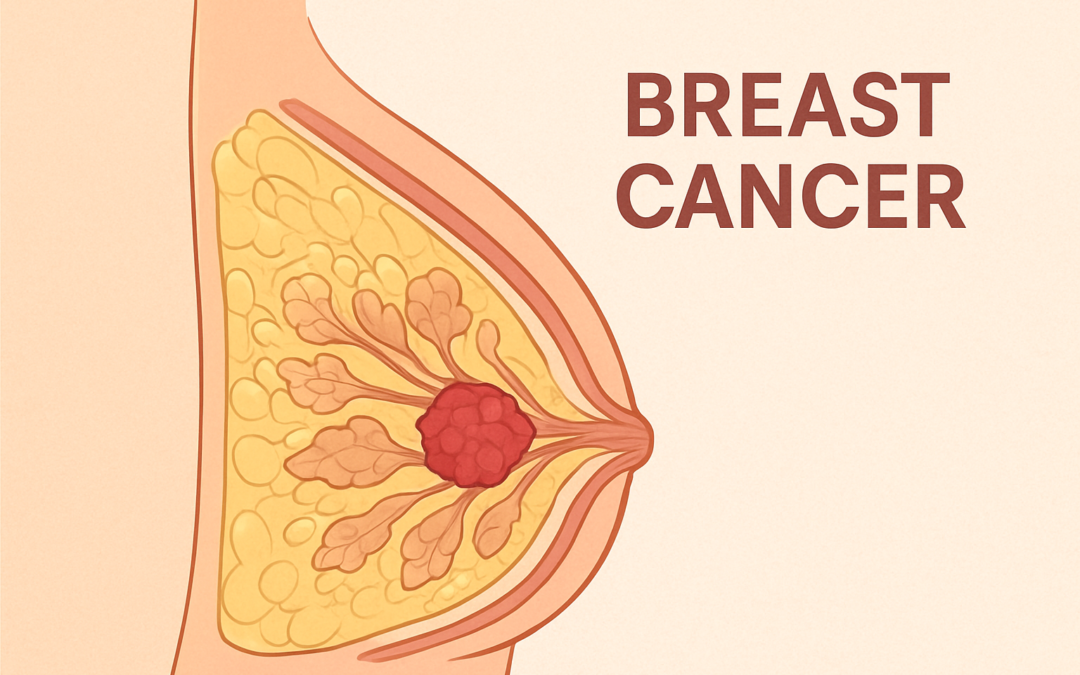
Eli Lilly and Company announced on September 25, 2025, that the U.S. Food and Drug Administration (FDA) has approved Inluriyo (imlunestrant), a 200 mg oral tablet, for the treatment of adults with estrogen receptor-positive (ER+), human epidermal growth factor receptor 2-negative (HER2-), ESR1-mutated advanced or metastatic breast cancer (MBC). This approval targets patients whose disease has progressed following at least one line of endocrine therapy (ET), addressing a critical need as approximately 50% of ER+, HER2- MBC patients develop ESR1 mutations after aromatase inhibitor exposure, leading to treatment resistance.

On September 25, 2025, the U.S. Food and Drug Administration (FDA) announced the approval of leucovorin calcium tablets for the treatment of cerebral folate deficiency (CFD), a neurological disorder characterized by impaired folate transport into the brain, resulting in developmental delays, autistic features (e.g., social communication challenges, sensory processing issues, repetitive behaviors), seizures, and motor coordination difficulties.

On September 22, 2025, the U.S. Food and Drug Administration (FDA) announced it is initiating a label change for acetaminophen, commonly known as Tylenol, to reflect evidence suggesting a potential association between its use during pregnancy and increased risks of autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) in offspring.







