
On August 6, 2025, the U.S. Food and Drug Administration (FDA) granted an expanded indication for AVTOZMA® (tocilizumab-anoh), a biosimilar to ACTEMRA® (tocilizumab), allowing its intravenous (IV) formulation to be used for the treatment of cytokine release syndrome (CRS) in adult and pediatric patients aged 2 years and older. This approval marks a significant milestone for Celltrion, Inc., the South Korea-based biopharmaceutical company behind AVTOZMA, as it now aligns the biosimilar with all FDA-approved indications for the reference product’s IV form.
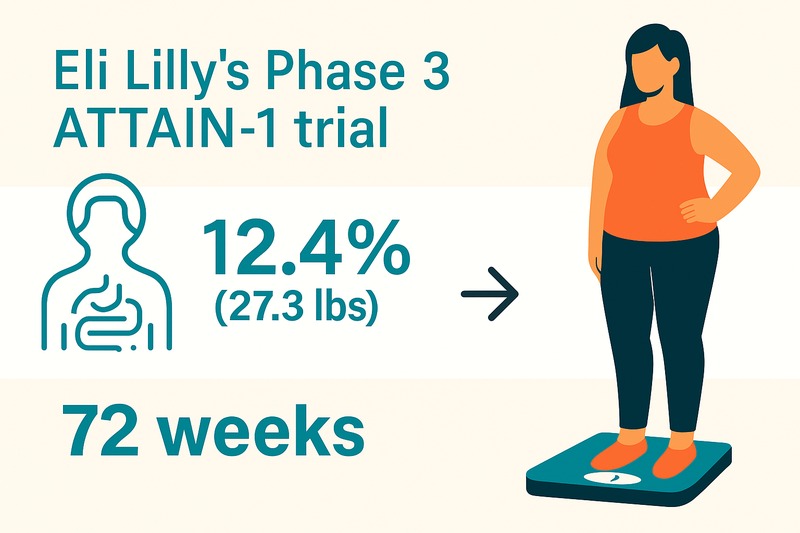
Obesity affects over one billion people globally, driving chronic disease burden. Orforglipron, an investigational oral glucagon-like peptide-1 (GLP-1) receptor agonist, offers a potential non-injectable treatment for weight management. The Phase 3 ATTAIN-1 trial evaluated its efficacy and safety in adults with obesity or overweight with weight-related comorbidities, excluding diabetes.
Revalesio, a clinical-stage biopharmaceutical company, has been granted Fast Track designation by the U.S. Food and Drug Administration (FDA) for its lead investigational therapy, RNS60, targeting acute ischemic stroke. This designation aims to expedite the development and review of RNS60, a proprietary oxygen-enriched saline designed to protect brain tissue following ischemic injury, addressing a critical gap in stroke treatment. Acute ischemic stroke, a leading cause of long-term disability and the second leading cause of death globally, often results in permanent neurological deficits despite advances in endovascular thrombectomy, which restores blood flow but does not prevent tissue damage post-reperfusion. Currently, no FDA-approved therapies exist to mitigate brain tissue loss during or after reperfusion, underscoring the urgent need for innovative treatments.

Gilead Sciences, Inc. announced that the U.S. Food and Drug Administration (FDA) has approved a supplemental New Drug Application (sNDA) for Biktarvy (bictegravir 50 mg/emtricitabine 200 mg/tenofovir alafenamide 25 mg tablets), expanding its indication to include treatment of people with HIV (PWH) who have an antiretroviral treatment (ART) history but are not virologically suppressed, provided they have no known or suspected resistance to the integrase strand inhibitor (INSTI) class, emtricitabine, or tenofovir.
The U.S. FDA has approved KERENDIA® (finerenone), a non-steroidal mineralocorticoid receptor antagonist (nsMRA), for treating adults with heart failure (HF) and left ventricular ejection fraction (LVEF) ≥40%. This approval follows a Priority Review and is based on findings from the Phase III FINEARTS-HF trial. The new indication allows KERENDIA to be used for reducing cardiovascular (CV) death, hospitalization for heart failure, and urgent heart failure visits in patients with mildly reduced (HFmrEF) or preserved ejection fraction (HFpEF).

The U.S. Food and Drug Administration has approved Yeztugo (lenacapavir), a powerful new HIV-prevention drug developed by Gilead Sciences. Given via injection just twice a year, Yeztugo has shown remarkable success in clinical trials, virtually eliminating HIV transmission among recipients. It significantly outperformed existing daily oral PrEP drugs like Truvada, cutting infection rates by 89% among gay and bisexual men and transgender individuals. In a separate trial among cisgender women in sub-Saharan Africa, none of the participants who received Yeztugo contracted HIV.







