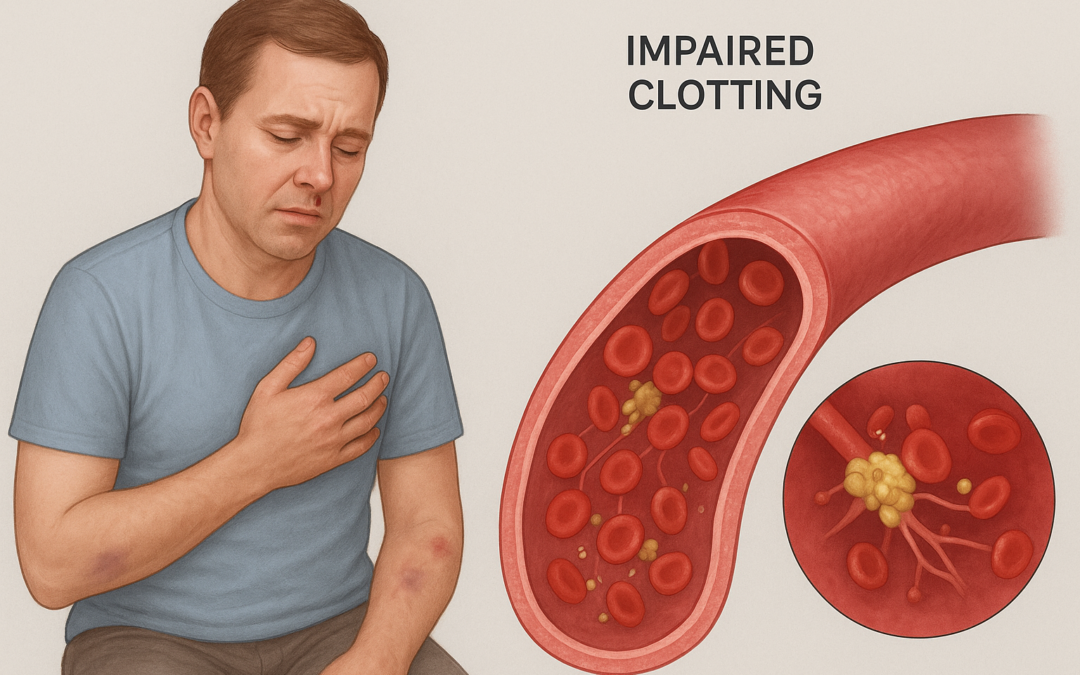
FDA Approves VONVENDI for Broader VWD Treatment in Adults and Children
On September 5, 2025, Takeda announced that the U.S. Food and Drug Administration (FDA) approved the supplemental Biologics License Application (sBLA) for VONVENDI® [von Willebrand factor (Recombinant)], expanding its indications to include routine prophylaxis for reducing bleeding episode frequency in adults with von Willebrand Disease (VWD), including Type 1 and 2, and on-demand and perioperative bleeding management in pediatric patients.

