The Global Consensus Recommendations for Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) and Metabolic Dysfunction-Associated Steatohepatitis (MASH), represent a landmark effort by an international multidisciplinary panel of 50+ experts from hepatology, endocrinology, and primary care. Developed via a two-round modified Delphi process (87% agreement threshold) and systematic literature review (up to May 2024), these guidelines update prior NAFLD/NASH frameworks to align with the 2023 nomenclature shift, focusing on cardiometabolic drivers to enhance diagnostic precision and therapeutic equity.
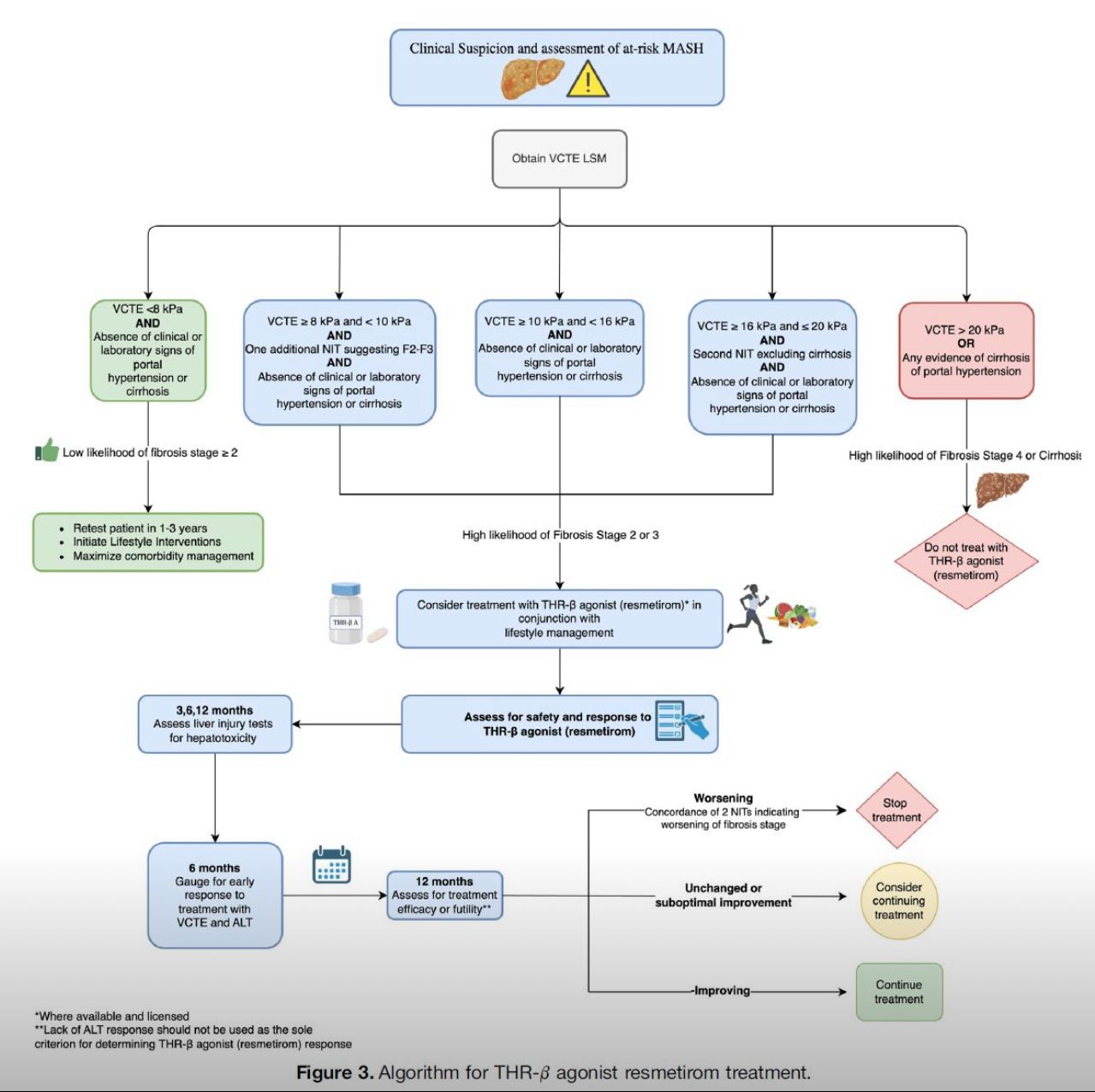
Objectives center on standardizing screening, diagnosis, staging, and management for MASLD—a continuum from simple steatosis (≥5% liver fat) to progressive MASH with inflammation/fibrosis—driven by insulin resistance and affecting ~38% of U.S. adults. Screening is recommended annually for high-risk groups (BMI ≥25, T2DM, metabolic syndrome) using FIB-4 or NAFLD Fibrosis Score; low-risk individuals require opportunistic evaluation. Positive screens prompt transient elastography (e.g., VCTE ≥8 kPa) or MRI-PDFF for fibrosis assessment, with biopsy reserved for diagnostic uncertainty or trial enrollment. Diagnosis mandates hepatic steatosis plus ≥1 cardiometabolic criterion (e.g., hypertension, dyslipidemia), excluding secondary causes. Staging employs the fibrosis classification (F0-F4), with non-invasive tools like ELF test or MRE preferred over biopsy for monitoring. Risk stratification integrates the AGILE score for hepatocellular carcinoma prediction in cirrhosis.
Management emphasizes personalized, stepwise approaches: foundational lifestyle modifications (Mediterranean diet, 150 min/week aerobic exercise) targeting 7-10% weight loss to reverse steatosis in 50-80% of cases. Pharmacologic options include pioglitazone/statins for non-cirrhotic MASH, semaglutide/tirzepatide for weight-related fibrosis, and resmetirom (FDA-approved 2024) for biopsy-confirmed MASH F2-F3. Bariatric surgery is endorsed for eligible obese patients. Follow-up intervals vary by stage (q6-12 months for advanced disease), incorporating HCC surveillance (US/MRI q6 months in cirrhosis).
Special considerations include pediatric screening from age 10 in obesity, pregnancy adaptations, and transplantation candidacy. The panel stresses multidisciplinary models, patient education, and health disparities mitigation. Limitations include reliance on expert opinion amid evolving data and regional variations in tool access. Future directions advocate for AI-enhanced biomarkers, phase 3 trials for combination therapies, and policy advocacy for liver fat screening in routine care. These recommendations aim to curb the projected 30% rise in MASH-cirrhosis by 2030, positioning MASLD as a modifiable epidemic through proactive, evidence-based strategies.
Link: https://www.gastrojournal.org/article/S0016-5085(25)00632-8/fulltext

