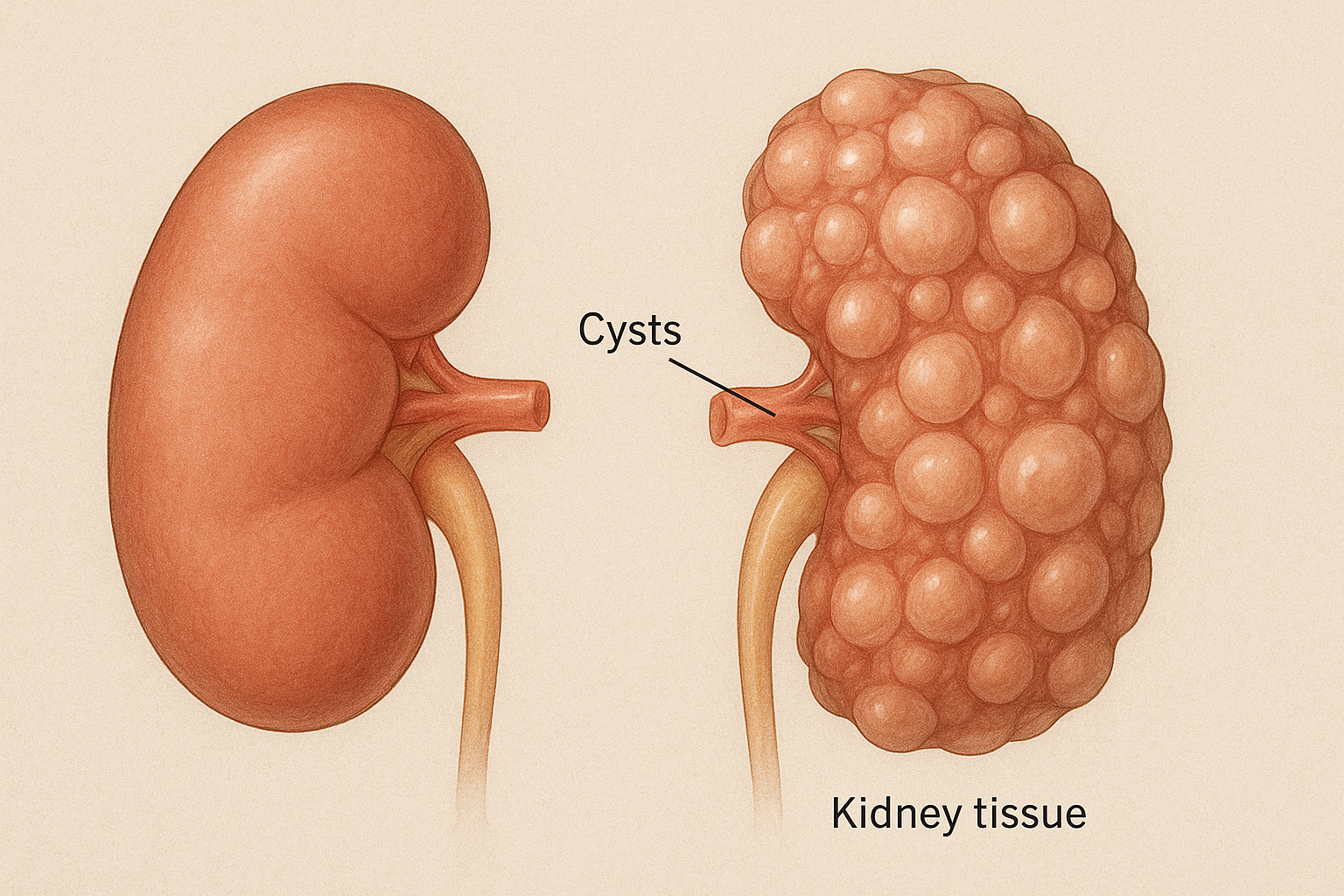
Autosomal dominant polycystic kidney disease (ADPKD) is a leading cause of kidney failure, necessitating novel therapies to slow disease progression. The Implementation of Metformin Therapy to Ease Decline of Kidney Function in Polycystic Kidney Disease (IMPEDE-PKD) trial is a prospective, multi-centre, international, double-blind, randomized, placebo-controlled phase III study evaluating metformin’s efficacy in reducing kidney function decline in ADPKD patients.
Targeting 1174 adults across kidney units in Australia, the UK, New Zealand, India, Hong Kong, South-East Asia, and Europe, the trial commenced recruitment in November 2022. Participants undergo a 10-week run-in phase with extended-release metformin, up-titrated to 2000 mg daily, followed by 1:1 randomization to metformin or placebo for 2 years.
The primary endpoint is the change in estimated glomerular filtration rate (eGFR), reflecting kidney function decline. Secondary endpoints include albuminuria, kidney failure onset, mortality, health-related quality of life, pain, medication tolerability, side effects, and cost-effectiveness. Emerging evidence suggests metformin may inhibit cyst growth, offering a potential, affordable, and accessible treatment for ADPKD. If effective, metformin could significantly improve patient outcomes by delaying dialysis and enhancing quality of life.
The trial’s robust design, global reach, and comprehensive outcome measures aim to provide definitive evidence on metformin’s role in ADPKD management, addressing a critical unmet need in this population.
Source: https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-025-09010-6

