
Diabetes mellitus is a chronic metabolic disorder with high global prevalence and considerable risk of long-term complications if glucose levels remain uncontrolled. Mobile health (mHealth) interventions—leveraging smartphones, SMS messaging, and telehealth technologies—offer scalable tools for diabetes self-management, patient engagement, and clinical monitoring. This systematic review and meta-analysis aimed to comprehensively evaluate both the clinical efficacy and cost-effectiveness of mHealth interventions in diabetes care.

Glomerular hyperfiltration at the single-nephron level often precedes detectable whole-kidney GFR decline in type 2 diabetes (T2D). Renal functional reserve (RFR), measured as the postprandial GFR increase, may unmask this hidden hyperfiltration. Sodium-glucose co-transporter-2 inhibitors (SGLT2i) like empagliflozin reduce intraglomerular pressure via tubuloglomerular feedback, often causing an initial GFR dip. We hypothesized that lower baseline postprandial RFR predicts greater empagliflozin-induced GFR reduction, but not responses to dipeptidyl-peptidase-4 inhibitor (linagliptin) or sulfonylurea.

Heart failure (HF) represents a prevalent and prognostically important complication in individuals with type 2 diabetes (T2D), contributing substantially to morbidity and mortality. Although glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have shown cardiovascular benefit in T2D, the impact of the once-daily oral semaglutide formulation on HF outcomes has not been fully characterized. The SOUL trial was a multinational, randomized, double-blind, placebo-controlled cardiovascular outcomes study in adults with T2D and atherosclerotic cardiovascular disease (ASCVD) and/or chronic kidney disease (CKD), originally designed to assess major adverse cardiovascular events (MACE). This secondary analysis evaluates the effect of oral semaglutide on HF outcomes according to HF status at baseline.
The phase 3 CORALreef Lipids trial, a multinational, double-blind, randomized, placebo-controlled study published in the New England Journal of Medicine on February 4, 2026, assessed the efficacy and safety of enlicitide decanoate, an investigational oral macrocyclic peptide inhibitor of proprotein convertase subtilisin/kexin type 9 (PCSK9). Enlicitide targets PCSK9 to increase hepatic LDL receptor availability and reduce circulating LDL cholesterol (LDL-C), offering a convenient oral alternative to injectable monoclonal antibodies.

On January 30, 2026, Indoco Remedies Ltd., a Mumbai-based integrated pharmaceutical company, received final approval from the United States Food and Drug Administration (USFDA) for its Abbreviated New Drug Application (ANDA) covering Lacosamide Oral Solution USP, 10 mg/mL. This generic formulation is bioequivalent and therapeutically equivalent to the reference listed drug (RLD), Vimpat Oral Solution 10 mg/mL, originally developed and marketed by UCB, Inc.

A retrospective cohort study published in Cureus on January 31, 2026, assessed the effectiveness of a specialized vascular risk clinic in Portugal in controlling major modifiable cardiovascular (CV) risk factors. Conducted between January 2022 and April 2023 (16 months), the analysis included 229 consecutive patients referred to the clinic, predominantly those with established atherosclerotic CV disease (ASCVD), diabetes, or high/very high CV risk per ESC/EAS guidelines. The multidisciplinary approach involved cardiologists, endocrinologists, nutritionists, and nurses, emphasizing intensive lifestyle counseling (diet, exercise, smoking cessation) alongside evidence-based pharmacotherapy titration (statins, antihypertensives, antiplatelet agents, GLP-1 agonists/SGLT2 inhibitors where indicated).
Gestational diabetes mellitus (GDM) is a common pregnancy complication associated with adverse maternal and fetal outcomes, including macrosomia, preterm birth, preeclampsia, and increased future diabetes risk. Myo-inositol, an insulin-sensitizing nutrient, has shown promise in prior studies for reducing GDM incidence in high-risk groups (e.g., those with family history of type 2 diabetes, obesity, or PCOS). This pilot trial, titled “Myo-Inositol for the Prevention of Gestational Diabetes Mellitus (MiGDM),” was designed as a randomized, double-blind, placebo-controlled study to preliminarily evaluate myo-inositol supplementation’s effects on GDM prevention and broader fetal/maternal outcomes.
Acute ischemic stroke (AIS) remains a leading cause of disability and death worldwide. The 2026 Guideline for the Early Management of Patients With Acute Ischemic Stroke, from the American Heart Association/American Stroke Association (AHA/ASA), replaces the 2018 guideline and 2019 focused update. It incorporates evidence from randomized controlled trials, meta-analyses, and observational studies published through early 2025, addressing prehospital systems, emergency evaluation, reperfusion therapies, supportive care, in-hospital complications, and early secondary prevention for adults, with new guidance extending to select pediatric cases.
On January 23, 2026, OKYO Pharma Limited (Nasdaq: OKYO), a clinical-stage biopharmaceutical company focused on therapies for neuropathic corneal pain (NCP) and inflammatory eye diseases, announced that the U.S. Food and Drug Administration (FDA) has authorized a single-patient expanded access Investigational New Drug (IND 176297) application—commonly known as compassionate use—for urcosimod (0.05%). The IND was submitted by Pedram Hamrah, MD, at the University of South Florida, to treat a patient suffering from severe NCP who has exhausted available therapeutic options, with no FDA-approved treatments currently existing for this condition.
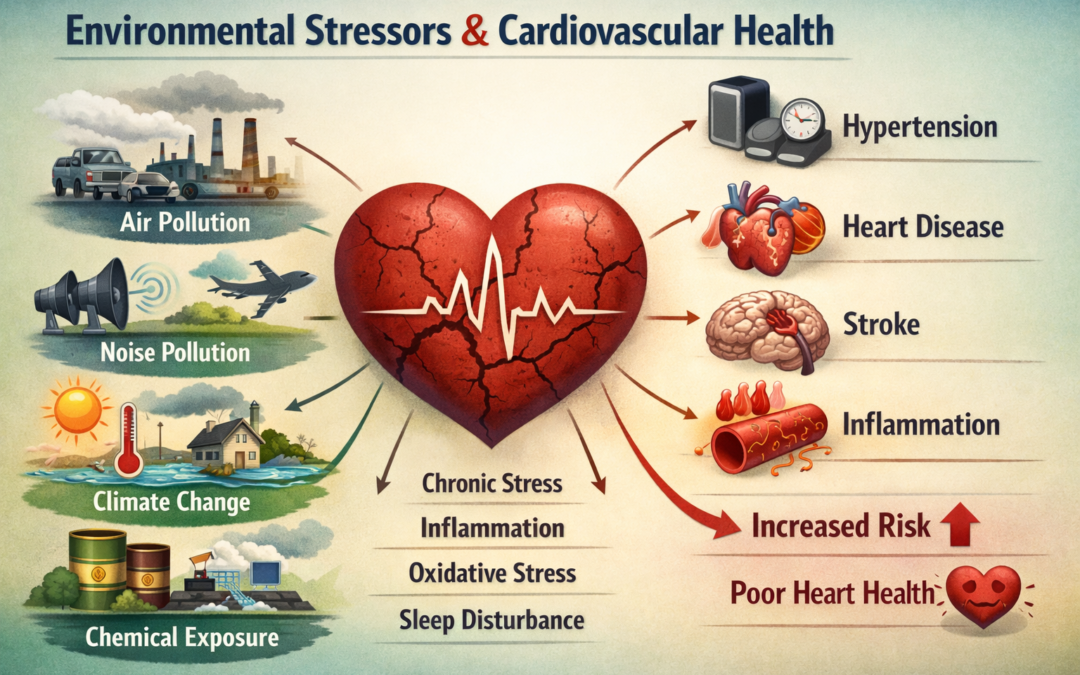
Non-communicable diseases (NCDs) account for 70% of global mortality, claiming over 38 million lives each year, with cardiovascular disease (CVD) as the leading contributor. While conventional risk factors like smoking, hypertension, and poor diet remain critical, emerging evidence highlights the escalating role of ubiquitous environmental risk factors (ERFs) in driving the rise of NCDs, particularly CVD. These interconnected anthropogenic exposures—air pollution, noise and light pollution, chemical and plastic contamination, water and soil pollution, and climate-related hazards—exert cumulative and compounding effects on cardiovascular health. They operate through shared pathophysiological mechanisms, including oxidative stress, systemic inflammation, autonomic nervous system imbalance, and endothelial dysfunction, amplifying overall risk beyond traditional factors.











