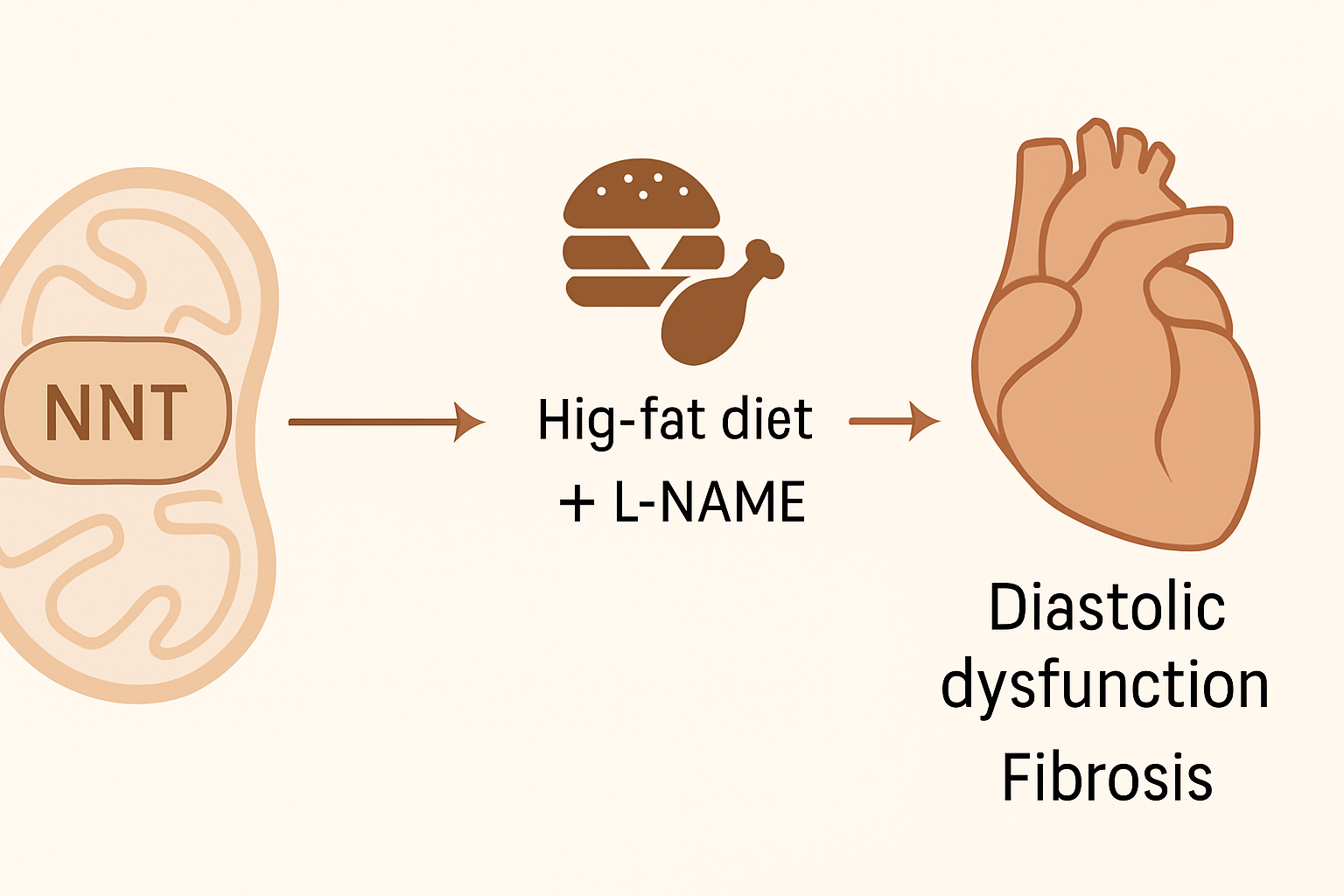
The study investigates the role of mitochondrial nicotinamide nucleotide transhydrogenase (NNT) in the development of heart failure with preserved ejection fraction (HFpEF), particularly in the context of cardiometabolic disease. HFpEF is a complex condition lacking effective therapies, and current preclinical models often rely on a “two-hit” approach—combining a high-fat diet with L-NAME (a nitric oxide synthase inhibitor)—to induce HFpEF-like features in mice.
Interestingly, the commonly used C57BL/6J mouse strain displays resistance to developing diastolic dysfunction under this model. This resistance is attributed to a naturally occurring loss-of-function mutation in the Nnt gene. NNT is an inner mitochondrial membrane protein involved in oxidative metabolism and redox balance by regulating the ratios of NADH/NAD⁺ and NADPH/NADP⁺, and maintaining glutathione homeostasis.
To directly test the role of NNT in HFpEF, researchers created isogenic C57BL/6N mouse models with either functional (Nnt+/+) or non-functional (Nnt−/−) Nnt and subjected them to the two-hit HFpEF model for 9 weeks. Nnt+/+ mice developed pronounced diastolic dysfunction, as indicated by increased E/e′, E/A ratios, diastolic stiffness, and myocardial fibrosis. In contrast, Nnt−/− mice were protected, showing significantly milder or absent pathological remodeling.
Biochemical analysis revealed that Nnt+/+ mice exhibited a 40% reduction in NAD⁺ and nearly 39% lower glutathione (GSH:GSSG) ratios, signifying mitochondrial redox imbalance. Single-nucleus transcriptomics and ligand-receptor signaling analysis pointed to fibroblast growth factor 1 (Fgf1) as a likely downstream mediator of NNT-driven fibrotic signaling from cardiomyocytes to fibroblasts.
In summary, this study identifies NNT as a critical driver of mitochondrial oxidative stress and redox imbalance that promotes diastolic dysfunction and fibrosis in HFpEF. The results highlight NNT and Fgf1 as promising targets for therapeutic intervention in HFpEF. Moreover, the findings demonstrate the importance of genetic background in preclinical modeling and suggest that therapies aimed at restoring redox homeostasis may be effective in treating HFpEF.
Source: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.125.326154

