Cerebral Blood Flow Is Lower in Youth with Type 2 Diabetes Compared with Obese Controls
At the 80th session of ADA, JACOB M. REDEL, presented his findings for cerebral blood flow in young T2DM patients compared with Obese Controls. As well established studies present a casual link between youth-onset type 2 diabetes (T2D) and vascular complications, the brain, as a highly vascular structure, may also be susceptible to the adverse effects of T2D. Brain structural and cognitive differences have been demonstrated in adolescents with T2D, and vascular disease is hypothesized to be a mechanism. The authors aimed to study the cerebral blood flow (CBF) in youth with T2D vs. obese, euglycemic controls, and to explore the association between CBF and a non-invasive measure of atherosclerosis, carotid intima medial thickness (IMT). Global and regional CBF between 20 youth with T2D were compared and 19 age, race, and sex similar obese controls, adjusted for socioeconomic status. arterial spin labeling (ASL) MRI was used to obtain CBF data. A comparison was conducted between mean CBF values, voxel-wise and globally averaged, between groups using two-sample t-tests. Voxel-wise results were evaluated for statistical significance (p<0.05) after adjustment for multiple comparisons.
In comparison to obese controls, the T2D group had significantly lower global CBF (49.7 ± 7.2 vs. 63.8 ±11.5 mL/gm/min, p<0.01). Seven voxel clusters with ≥100 voxels identified with significantly lower CBF in the T2D group, and no clusters with higher CBF. In the T2D group, IMT of the common carotid artery was inversely correlated with CBF, both globally (r= -0.7, p<0.01) and in regional voxel clusters. The study results thus suggested that global and regional cerebral blood flow is lower in youth with T2D compared to obese controls. Further work is needed to determine whether these CBF findings are related to brain structural or cognitive differences previously reported in youth-onset T2D.
Conclusion: The association found between carotid IMT and CBF suggests this may represent a practical way to track CBF in youth with T2D.

Diastolic Dysfunction, a Precursor for Heart Failure in Young Adults with Youth-Onset Type 1 and Type 2 Diabetes: The SEARCH for Diabetes in Youth Study
AMY S. SHAH, et.al. presented their findings at the 80th session of ADA. T2DM patients are at a higher risk for heart failure with preserved ejection fraction due to diabetic cardiomyopathy (DCM), defined as abnormal myocardial structure and function in the absence of overt coronary artery disease. DCM leads to myocardial fibrosis and remodeling whose initial symptom is left ventricular (LV) diastolic dysfunction. The objective of the study was to compare the prevalence of diastolic dysfunction among young adults with type 1 diabetes (T1D) vs. type 2 diabetes (T2D) with diabetes diagnosed in childhood. The Youth Study comprised of participants having T1D or T2D. Cardiovascular risk factors and diastolic function were measured at an in-person visit via echocardiography, after an average disease duration of 10.9 years (both groups). The study defined diastolic dysfunction as abnormal LV filling volume, LV pressure, or transmitral velocity, based on published norms for age. Of 458 eligible participants, 255 had T1D, and 203 had T2D. Study participants with T2D showed a worse cardiovascular risk profile (higher BMI, systolic and diastolic BP, triglycerides, LDL-C, A1c and lower HDL-C) than those with T1D, all p<0.05. They also had lower LV filling volume, transmitral velocity and higher LV pressure, all p<0.05, than those with T1D, suggesting worse diastolic function. Both groups depicted high unadjusted prevalence of diastolic dysfunction (57.7% in T2D vs. 47.2% in T1D; p<0.05).
Conclusion: In comparison to T1D patients, T2D shows worst diastolic function but diastolic dysfunction is high in both groups. These findings support monitoring of young adults with diabetes for development of heart-related complications.

Glycaemia, Beta-Cell Function, and Incretin Hormones Post-OGTT One Year after Gastric Bypass and Sleeve Gastrectomy in Patients with Morbid Obesity and Type 2 Diabetes: An RCT (Oseberg Study)
FARHAT FATIMA et.al presented the Oseberg Study at the 80th session of ADA. The study demonstrated a higher one year remission rate of type 2 diabetes after Roux-en-Y gastric bypass (RYGB) than after sleeve gastrectomy (SG).
The authors, in order to explore mechanisms for the superiority of RYGB; assessed changes in glucose, C-peptide and incretin hormones after a 25 g OGTT and an IVGTT and calculated Disposition Indexes (DI) of beta cell function (DI and DI ). Linear mixed models for repeated measures were used. Patients randomized to either RYGB (n=54) or SG (n=55), had a mean (SD) age of 48 (10) years, BMI 42.3 (5.3) kg/m², HbA 66 (18) mmol/mol, and 72 (66%) were women. OGTT and IVGTT were performed in 97% and 91% of patients respectively. The OGTT-derived glucose levels at 60-120 minutes were significantly lower after RYGB than after SG (all p<0.05) at one year. The OGTT-derived increase in measures of C-peptide, GIP and GLP-1 from 0 to 15 minutes were significantly higher in the RYGB group, mean (95% CI) between group difference: C-peptide 257 (66, 448) pmol/L, p=0.008; GIP 11 (4, 19) pmol/L, p=0.005; and GLP-1 29 (20, 38) pmol/L, p<0.001. A three to five fold increase of DI and DI was observed in both groups with no significant difference between groups.
Conclusion: Post- OGTT glucose levels were significantly lower after RYGB as compared with SG, possibly mediated by a greater increase in GIP, GLP-1 and C-peptide levels.

Trends in Complications in Type 1 and Type 2 Diabetes in Denmark, 1996-2016
At the 80th session of ADA, BENDIX CARSTENSEN et.al. described the burden and trend in 18 groups of complications in persons with diabetes in Denmark 1996-2016. The authors, compiled a diabetes register for the entire Danish population, including reliable classification of type of diabetes.
The author extracted dates of recorded complications in 18 classes from the National Patient Register. Binomial regression was used to describe the prevalence of complications at diagnosis and change in these over time. In order to describe the incidence rates, Rate models based on Poisson likelihood were used. Analyses were conducted separately for T1D and T2D, and controlled for sex, age, duration of diabetes and including a linear term to describe the calendar time effect (date of diagnosis).
T1D showed 10% and T2D showed 37% of prevalence at diagnosis of previous CVD with annual relative changes of 3% in T1D and 5% in T2D. Retinopathy prevalence at diagnosis was 4.3% in T1D and 1.7% in T2D with no change in T1D, but a relative decrease of 6% in T2D. Similar decreases in rates of complications in T1D and T2D; decrease of 2%/year in rates of CVD, 5%/year in amputations, and no changes in the incidence rates of retinopathy and renal disease over the period 1996-2016 were found by the authors. For most complications the authors found a very high incidence during the first year after diagnosis, most likely reflecting a diagnostic catch-up and not any biological phenomenon.
Conclusions: A steady decrease in incidence rates of most complications was seen, but not for microvascular complications. The pattern of complications at diagnosis was more mixed, influenced by fluctuations in diagnostic activity over the period. In general an encouraging trend in complication occurrence was seen in Denmark in the period 1996-2016.

Diabetes-Related Quality of Life Assessment in Children following Total Pancreatectomy with Islet Autotransplantation
JACOB M. REDEL presented at the 80th scientific session of ADA for the topic of Diabetes Related Quality of Life Assessment in Children following Total Pancreatectomy with Islet Autotransplantation.
Pain and functional impairment associated with chronic pancreatitis (CP) in children can effectively be improved by total pancreatectomy with islet autotransplantation (TPIAT). However, children undergoing this procedure usually develop post-pancreatectomy diabetes and require chronic insulin therapy. It can be difficult for providers and patients to conceptualize trading the symptoms of CP for the burden of diabetes. Thus the authors sought to evaluate diabetes-related quality of life (QOL) over time in children who underwent TPIAT, and compare QOL scores post-TPIAT with children who have type 1 diabetes (T1D). Diabetes-related QOL was assessed using Pediatric Quality of Life Inventory (PedsQL) 3.2 Diabetes Module. PedsQL scores were evaluated in 46 children (<20 years old) who underwent TPIAT and completed at least one survey. PedsQL data was collected at 3, 6, 9, 12, 18, and 24 months post-TPIAT. Total and Subscale scores were analyzed for change over time. The TPIAT cohort PedsQL scores at 24 months (n=16) were then compared to PedsQL scores from a historical cohort of demographically similar (age, race, sex) patients 24 months after new onset T1D (n=58). Mean age at TPIAT was 12.6 ± 4.7 years and 67% were female. Mean age at T1D diagnosis was 11.2 ± 3.2 years and 69% were female. The Diabetes Symptoms subscale (p=0.03) and the Total Score (p=0.049) decreased (worsened) over 24 month’s post-TPIAT (Diabetes subscale: median 68 to 56; Total Score: median 75 to 68). The other subscales did not significantly change over that time. At 24 months after diabetes onset, no PedsQL subscales were different between the TPIAT cohort and T1D cohort (all p>0.2). The Total PedsQL score, mostly attributable to the Diabetes Symptoms subscale, decreased over time in patients who underwent TPIAT. Additionally, diabetes-related QOL at 24 months was not significantly different from children with T1D.
Conclusion: The data from the study may be used to help counsel families who are considering TPIAT for treatment of CP.
Effects of Intensive Risk Factor Management on Cardiovascular Autonomic Neuropathy in Type 2 Diabetes: Findings from the ACCORD Trial
YALING TANG presented the findings from the ACCORD Trial at the 80th scientific session of ADA. In patients with Type 2 Diabetes (T2D), cardiovascular autonomic neuropathy (CAN) is a common complication that independently predicts cardiovascular (CV) morbidity and mortality.
The effect of preventive interventions on CAN remains unclear. The authors examined the effects of intensively targeting hyperglycemia, hypertension, and dyslipidemia on CAN, in persons with T2D and high CV risk from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. CAN was defined as heart rate variability indices below the 5 percentile of the normal distribution (standard deviation of all normal-to-normal R-R intervals [SDNN] <8.2 ms and root mean square of successive differences between normal-to-normal R-R intervals [rMSSD] <8.0 ms). From the 10,251 ACCORD participants, 71% (n=7,275) had valid CAN measures at study entry and at least once during follow-up. As compared to standard treatment, intensive glycemic control had a protective effect on CAN (OR=0.83, 95% CI 0.74 – 0.93, p=0.002), especially in persons with no CVD history (OR= 0.72, 0.62 – 0.83, p<0.0001). Intensive BP therapy also decreased the odds of CAN (OR=0.82, 0.72 – 0.94, p=0.01), especially in persons with positive CVD history (OR=0.71, 0.53 – 0.96, p=0.03). Fenofibrate did not have a significant impact on the dichotomous CAN outcome but showed a significant benefit on continuous CAN outcomes SDNN and rMSSD. No significant interactions were observed between treatments.
Conclusion: As per the authors, this study was the first to demonstrate a clear benefit of intensive glycemic and BP control, and fenofibrate therapy on CAN in a large T2D cohort and high CV risk. The finding of possible heterogeneity in the benefit of these interventions on CAN across clinical strata suggests personalization of these treatments as a path forward to optimize their use.

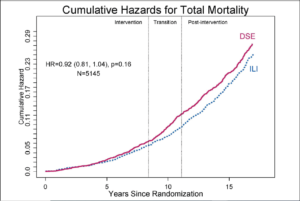
All-Cause Mortality over 16 Years in Look AHEAD
RENA R. WING presented on All-cause mortality over 16 years at the 80th scientific session of ADA. Reduction in mortality in overweight/obese individuals with diabetes due intentional weight loss is unclear. Look AHEAD, a randomized trial of intensive lifestyle intervention (ILI) and diabetes support and education (DSE) (control) in 5145 individuals with overweight/obesity and type 2 diabetes, is ideal for examining this due to large sample, extended follow-up, excellent retention, and sustained difference in weight loss (y1: 8.6% ILI; 0.7% DSE; y10: 6.1% ILI; 3.9% DSE). In the study, after 10 years of intervention, participants had 6-month calls and biennial visits. The main outcome for the current phase was all-cause mortality from randomization to 16 years. Proportional hazards regression stratified by clinical site were used to analyze data; significance was based on the likelihood ratio. All-cause mortality was 9% lower in ILI compared to DSE (Fig 1). Deaths by arm were: ILI 496/2570 and DSE 530/2575. Interactions of treatment with prespecified subgroups (sex, CVD history, age) were not significant. In post-hoc analyses, ILI participants who lost >10% at 1year had an 18% reduced risk (HR=.82, CI 0.68,0.97) and ILI participants who gained or lost <2% had a 29% higher risk (HR=1.29, CI 1.01,1.67) relative to all DSE. The difference in mortality between ILI and DSE was not statistically significant.
Conclusion: Post-hoc analyses suggest an association between magnitude of weight change and all-cause mortality in ILI.
Empagliflozin Improves Insulin Sensitivity of the Hypothalamus in Humans with Prediabetes
MARTIN HENI presented a study on Improvement in Insulin sensitivity with Empagliflozin in humans with prediabetes. Insulin acts on the human brain and reduces food intake, improves whole-body insulin sensitivity, and modulates adiposity. In most cases of obesity and diabetes, the brain becomes insulin resistant with impaired brain-derived modulation of peripheral metabolism. As treatment with the SGLT2-inhibitor empagliflozin not only improves glucose metabolism but also reduces body weight and cardiovascular risk, the authors hypothesized that improved brain insulin sensitivity could be involved. A double blind study was conducted by the authors. 40 participants with prediabetes (according to ADA’s OGTT criteria) were 1:1 randomized to receive 25 mg empagliflozin qd or placebo (mean ± SD: age: 60 ± 9 years; BMI: 31.5 ± 3.8 kg/m²). Before and after 8 weeks of treatment, brain insulin sensitivity was assessed by functional MRI combined with the intranasal administration of insulin to the brain. In healthy persons, intranasal insulin administration significantly decreases cerebral blood flow in the hypothalamus. In this study the volunteers with prediabetes were unresponsive to this, as insulin could not induce hypothalamic inhibition prior treatment. The authors thus identified a significant interaction between treatment and the hypothalamic response to insulin (p<0.05, corrected for multiple comparisons). Post-hoc analyses showed that only participants on empagliflozin showed a significant insulin-induced decrease in hypothalamic blood flow after treatment. The group receiving placebo showed no such improvement.
Conclusion: The study results corroborate insulin resistance of the human hypothalamus in humans with prediabetes. Treatment with empagliflozin for 8 weeks was able to restore hypothalamic insulin sensitivity; a favorable response that could contribute to the positive effects of SGLT2 inhibitors. These findings reveal that brain insulin resistance is treatable by pharmacological interventions with potential benefits for cognition, adiposity, and whole-body metabolism.
ADA Presidents’ Select Abstract: Symptom Responses to Hypoglycemia Are Related to Connectivity Change between the Thalamus and Frontal Lobes
PETER JACOB presented his study at the the 80th scientific session of ADA. In people with or without type 1 diabetes, neuroimaging studies highlight the importance of thalamic activation in response to hypoglycemia. This activation is characteristically attenuated in impaired awareness of hypoglycaemia (IAH), a major risk factor for severe hypoglycemia. The authors investigated changes in connectivity of the thalamus with other brain regions during hypoglycemia. In the study, fourteen people without diabetes (no diabetes, ND), 15 with T1D and normal awareness of hypoglycemia (NAH) and 22 with IAH, all right-handed, age, sex and gender matched, underwent a hyperinsulinemic glucose clamp during which the authors acquired blood oxygen level dependent fMRI at euglycemia (90mg/dL) and at onset of hypoglycemia (48mg/dL). CONN toolbox in SPM12 was used to study connectivity. A thalamic seed was used in a seed-tovoxel analysis with and without the increase in symptoms at hypoglycemia as an explanatory regressor variable.
The study results showed that symptom scores in response to hypoglycemia rose earliest and to the greatest levels in NAH (11.0 to 18.3, p= 0.029). The rise in symptom scores in ND was not significant at this timepoint (9.2 to 13.8, p= 0.057), whilst IAH showed no symptom change (10.5 to 9.8, p= 0.7). In ND, hypoglycemia increased thalamic connectivity with the right frontal pole (p = 0.018). This did not occur in diabetes, either in NAH or IAH. The NAH group demonstrated a positive relationship between change in symptoms with change in connectivity of the thalamus and a left frontal pole region (p = 0.035), whereas an overlapping area had a negative correlation in ND (p = 0.001). There was no correlation in IAH.
Conclusion: During hypoglycemia, those with T1D display aberrantly enhanced thalamic connectivity with brain regions responsible for complex behavior. Disruption of this network occurs in IAH and may be the mechanism for failure of perception of the onset of hypoglycemia and reduced hypoglycaemia avoidance behaviors, increasing severe hypoglycemia risk.

