Tirzepatide, a dual GLP-1 and GIP receptor agonist, has been shown to improve glycaemic control and promote weight loss when compared with selective GLP-1 receptor agonists. Benefits of tirzepatide have also been observed for atherogenic lipoproteins, blood pressure, high sensitivity C-reactive protein and kidney function in comparison with selective GLP-1 receptor agonists or basal insulins. Stephen Nicholls assessed the cardiovascular outcomes in participants on tirzepatide versus dulaglutide in the SURPASS-CVOT trial. The findings were presented at the EASD Annual Meeting 2025, held th st from 15- 19 September 2025 in Vienna, Austria.
Over 2 years, 13,299 people at 640 sites in 30 countries across all world regions were randomized. The mean age of randomized participants at baseline was 64.1 years, diabetes duration 14.7 years, HbA1c 8.4%, and BMI 32.6 kg/m2. Overall, 65.0% had coronary disease, of whom 47.3% reported prior myocardial infarction and 57.4% had prior coronary revascularization. 19.1% of participants had a prior stroke and 25.3% had peripheral artery disease. The trial is fully recruited and ongoing.
Over 2 years, 13,299 people at 640 sites in 30 countries across all world regions were randomized. The mean age of randomized participants at baseline was 64.1 years, diabetes duration 14.7 years, HbA1c 8.4%, and BMI 32.6 kg/m2. Overall, 65.0% had coronary disease, of whom 47.3% reported prior myocardial infarction and 57.4% had prior coronary revascularization. 19.1% of participants had a prior stroke and 25.3% had peripheral artery disease. The trial is fully recruited and ongoing.
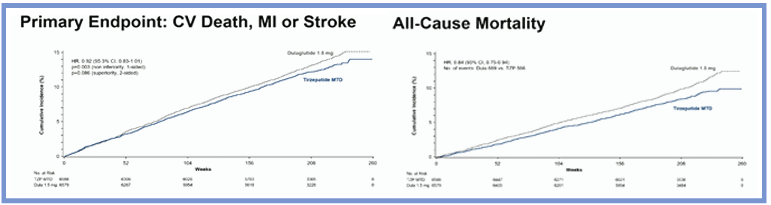
Tirzepatide had favourable effects on HbA1c, body weight, change in eGFR, all-cause death and the composite outcome of cardiovascular death, myocardial infarction, stroke or coronary revascularisation compared with dulaglutide. Safety outcomes were similar for participants who received tirzepatide compared with dulaglutide; however, an increase in gastrointestinal adverse events was observed in the tirzepatide group compared with dulaglutide. Treatment with tirzepatide met the pre-specified criteria for non-inferiority compared with dulaglutide on the rate of the primary composite endpoint of cardiovascular death, myocardial infarction or stroke. Tirzepatide met the criteria for superiority compared with a putative placebo on the rate of the primary composite endpoint of cardiovascular death, myocardial infarction or stroke.
Overall, SURPASS-CVOT demonstrated that tirzepatide is cardioprotective in participants with established atherosclerotic cardiovascular disease and type 2 diabetes. This active comparator study established that tirzepatide had favourable clinical effects for high-risk participants with type 2 diabetes and atherosclerotic cardiovascular disease, and could provide an additional incretin-targeted therapy for cardiovascular prevention in the setting of type 2 diabetes.

