
On January 30, 2026, Indoco Remedies Ltd., a Mumbai-based integrated pharmaceutical company, received final approval from the United States Food and Drug Administration (USFDA) for its Abbreviated New Drug Application (ANDA) covering Lacosamide Oral Solution USP, 10 mg/mL. This generic formulation is bioequivalent and therapeutically equivalent to the reference listed drug (RLD), Vimpat Oral Solution 10 mg/mL, originally developed and marketed by UCB, Inc.
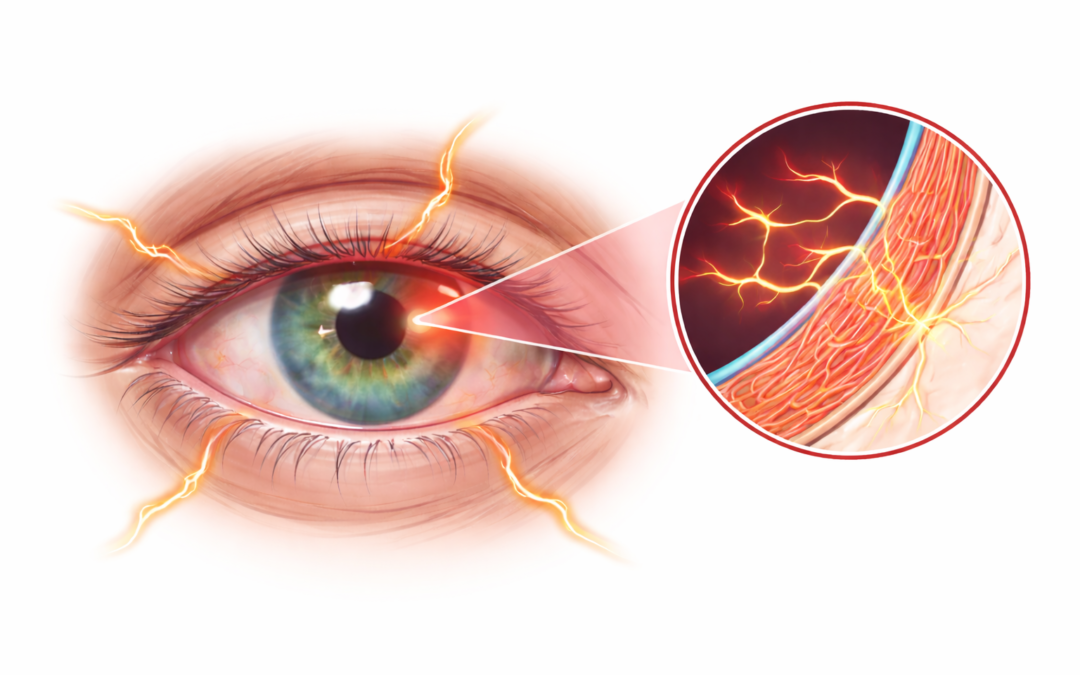
On January 23, 2026, OKYO Pharma Limited (Nasdaq: OKYO), a clinical-stage biopharmaceutical company focused on therapies for neuropathic corneal pain (NCP) and inflammatory eye diseases, announced that the U.S. Food and Drug Administration (FDA) has authorized a single-patient expanded access Investigational New Drug (IND 176297) application—commonly known as compassionate use—for urcosimod (0.05%). The IND was submitted by Pedram Hamrah, MD, at the University of South Florida, to treat a patient suffering from severe NCP who has exhausted available therapeutic options, with no FDA-approved treatments currently existing for this condition.

On January 4, 2026, Ascletis Pharma Inc. (HKEX: 1672), a Hong Kong-listed biotechnology company specializing in metabolic diseases, announced U.S. FDA clearance of its Investigational New Drug (IND) application for a Phase II clinical trial of ASC30 in type 2 diabetes mellitus (T2DM). ASC30 is an in-house discovered oral small molecule glucagon-like peptide-1 receptor (GLP-1R) fully biased agonist, designed for once-daily oral administration or once-monthly to once-quarterly subcutaneous dosing, targeting obesity, diabetes, and related conditions.

On January 5, 2026, ScinoPharm Taiwan Ltd. (TWSE: 1789), based in Tainan, announced a significant milestone: U.S. Food and Drug Administration (FDA) approval for its Glatiramer Acetate Injection, a therapeutic equivalent to Teva’s Copaxone® for the treatment of relapsing forms of multiple sclerosis (MS). This approval positions ScinoPharm as the only Taiwanese pharmaceutical company to successfully navigate the stringent regulatory pathway for this product, underscoring Taiwan’s emerging role in the global complex generics sector.

Alpha- and beta-thalassemia are inherited hemoglobinopathies causing chronic hemolytic anemia, ineffective erythropoiesis, iron overload, and complications including fatigue and organ damage. Management has relied on supportive care such as transfusions and chelation, with no prior disease-modifying oral therapies approved for all subtypes. Mitapivat, a first-in-class oral pyruvate kinase activator, targets underlying red blood cell energy deficits to improve survival and function.

On December 30, 2025, Vanda Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) approved NEREUS™ (tradipitant), an oral neurokinin-1 (NK-1) receptor antagonist, for the prevention of vomiting induced by motion in adults. This approval represents a historic milestone, as it introduces the first new pharmacologic treatment for motion sickness in over 40 years, advancing beyond traditional antihistamines and antimuscarinics like scopolamine patches, which often carry limitations such as drowsiness or limited efficacy.







