On October 20, 2025, the U.S. Food and Drug Administration (FDA) granted approval to Roche’s Gazyva/Gazyvaro (obinutuzumab) for the treatment of lupus nephritis (LN), a severe complication of systemic lupus erythematosus (SLE) that affects the kidneys. Lupus nephritis is characterized by inflammation that can lead to kidney damage, impaired function, and, in severe cases, kidney failure.
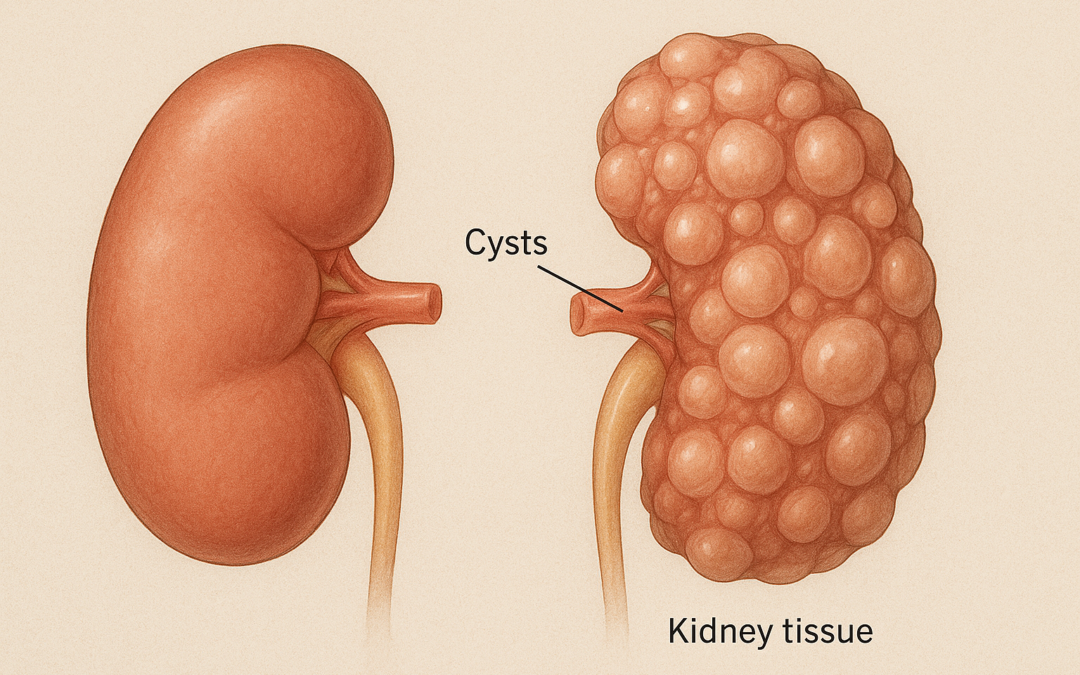
Autosomal dominant polycystic kidney disease (ADPKD) is a leading cause of kidney failure, necessitating novel therapies to slow disease progression. The Implementation of Metformin Therapy to Ease Decline of Kidney Function in Polycystic Kidney Disease (IMPEDE-PKD) trial is a prospective, multi-centre, international, double-blind, randomized, placebo-controlled phase III study evaluating metformin’s efficacy in reducing kidney function decline in ADPKD patients.
Fendo is a pain-relieving medicine that contains Diclofenac Sodium, a widely used ingredient for reducing pain and inflammation. It works by blocking certain chemicals in the body responsible for swelling and discomfort, making it particularly useful for conditions such as arthritis, muscle pain, back pain, and post-surgical recovery.
This 2025 review highlights the manner in which combination therapy has become the preferred treatment for chronic kidney disease (CKD) in diabetic and non-diabetic patients. CKD treatments have recently come up with new drugs that not only protect the kidneys but also lower the risk of cardiovascular complications and kidney failure.
The article discusses the potential use of targeted-release budesonide (TRF-budesonide) in treating IgA nephropathy (IgAN), a common kidney disease characterized by IgA deposits in the kidneys. IgAN can lead to end-stage renal disease, and current treatments like ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) aren’t always effective in slowing disease progression.

The FDA has approved Novo Nordisk’s Ozempic to treat chronic kidney disease (CKD) in patients with Type 2 diabetes, expanding its role beyond diabetes management. Previously used primarily for blood sugar control and weight loss, Ozempic can now help slow kidney function decline, reduce the risk of kidney failure, and lower the chances of death from cardiovascular disease in these patients.







