Insulin is highly sensitive to degradation from stomach acid and enzymes, but quantum dot (QD) conjugation can help make it insoluble across a wider pH range. While QD-insulin conjugation improved stability, QD-insulin alone was not effective in mice studies. The findings were presented at the EASD Annual Meeting 2025, held from 15 -19 September 2025 in Vienna, Austria.
A recent 2024 study by Hunt NJ, et al, stated that oral insulin shows physiological effects comparable to 2 IU/kg injected insulin, requiring ~20 IU/kg orally. At hypoglycemic doses, 5 IU/kg injected insulin is equivalent to ~500 IU/kg oral insulin. Moreover, controlled insulin release from CS/GS occurs via hydrolysis and enzymatic degradation, with cellulase and glucosidase driving rapid release and glucosidase enabling glucose-linked linear release. While QD-Insulin CS/GS showed dose-dependent glucose reduction, with 10–30% decreases at 2–10 IU/kg doses in non diabetic baboons. Subgroup analysis in non-diabetic baboons showed baseline BGL at 4.9 or 2.9. No effect was observed in the hypoglycemia-risk group. A 10% reduction was seen in the normoglycemia group. Oral insulin exhibited advantages, as it acts rapidly, shows dose-dependent effects and in early diabetic models, and showed comparable results to injected insulin. Pharmacodynamic testing confirmed dose-dependent glucose reduction across mice, rats, and baboons.
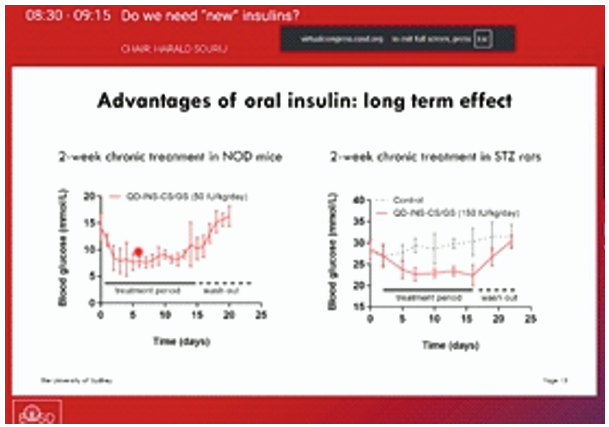
Two-week oral insulin treatment in NOD mice and STZ rats significantly lowered blood glucose, with levels rising again after washout. In human duodenum explants, QD-INS-CS/GS conjugation increased insulin uptake by 40-fold compared to insulin alone.
Oral insulin must overcome physical and chemical barriers and its uptake can be improved using zwitterionic polymers. Oral insulin offers the advantage of reducing hypoglycemia risk, and is expected to enter clinical trials in 2026.

