Analyses of First Plus Recurrent Cardiovascular (CV) and Hospitalization Events in the CAROLINA Trial
Nikolaus Marx presented Analyses of First plus Recurrent Cardiovascular (CV) and Hospitalization Events in the CAROLINA Trial on Friday June 12, 2020 at American Diabetes Association (ADA) 80th Scientific Sessions, a Virtual Experience. As per the trial linagliptin was found to be safe in patients with type 2 diabetes and related complications. The CAROLINA Trial was a randomized controlled clinical trial performed to evaluate comparative effects of linagliptin with glimepiride on cardiovascular (CV) events and other outcomes in patients with relatively early type 2 diabetes (T2D) with elevated CV risk.
A negative Binomial model was used to assess the effects of linagliptin and glimepiride on all first CV event and recurrent CV events along with any-cause hospitalizations. About 6033 participants were enrolled in the trial. Baseline characteristics were mean age 64.0 years, HbA1c 7.2%, BMI 30.1 kg/m2, eGFR 77 ml/min/1.73m2. The median T2D duration was 6.3 years and UACR was 9 mg/g. 42% patients had CV disease, while 4.5% patients had heart failure [HF].
The number of events from first event were increased by 10% to 77% across CV/HF outcomes and by 119% for hospitalizations with corresponding increase in rates per 100-patient years in both treatment groups. (3P-MACE from 2.1 to 2.8 [Linagliptin] and 2.1 to 2.9 [Glimepiride], over median 6.3 years). 4P-MACE events increased from 2.3 to 3.2 in linagliptin group comparable with that of glimepiride were increase was observed from 2.4 to 3.3.
Likewise, all-cause hospitalizations first to all events increased from 9.2 to 15.9 and 9.8 to 17.1 in linagliptin and glimepiride groups, respectively.
Conclusion: Overall, Linagliptin or Glimepiride showed no significant differences for either first or recurrent CV or hospitalization events. Significant CV disease burden was experienced even in relatively early T2D and established the safety of linagliptin.

Blood Pressure as Predictor of Coronary Artery Disease (CAD)/Cerebrovascular Disease (CVD) According to Glucose Tolerance Status (GTS): Implications for Updated Guidelines
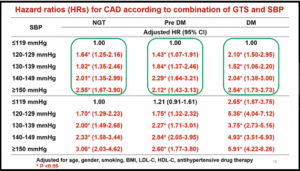
Guidelines according to GTS remain to be a topic of debate in spite of recent tightening of cutoffs for hypertension (HT) in major global guidelines. MAYUKO H. YAMADA and team analyzed data from a nationwide claims database on 293,396 participants without prior CAD or CVD from 2008-16. Risks of CAD and CVD events were analyzed by Multivariate Cox regression model. Hazard ratios (HRs) were compared among 5 levels of systolic blood pressure (SBP) (≤119, 120-129, 130-139, 140-149, ≥150) according to GTS [i.e., normal glucose tolerance (NGT), prediabetes (pre-DM), diabetes (DM)].
The study period showed 1099 CAD and 1990 CVD events. A significantly higher risk of incident CAD/CVD was observed in patients with SBP levels ≥120 mmHg than those with lower levels. The incidence of CAD and CVD did not differ significantly between NGT and pre-DM in those with SBP ≤119 whereas HRs for incident CAD for DM was almost 2.7, which was similar to NGT and pre-DM with SBP ≥150 mmHg (Table).
In comparison to NGT and SBP ≤119 mmHg, the HR for incident CAD among DM and SBP ≥150 mmHg was 5.91 (4.22-8.26), while SBP ≥150 mmHg notably increased the risk of CVD about 4 fold regardless of GTS.
Conclusion: These results based on a large database implied the need for even lower BP targets regardless of GTS.
Implementation of an Intensive Telehealth Intervention for Rural Patients with Uncontrolled Diabetes
A research was presented by ELIZABETH A. KOBE et.al. At the 80th Scientific Sessions A Virtual Experience on 12th June 2020 which exhibited rural implementation of an intensive telehealth intervention designed to leverage existing Veterans Health Administration (VHA) Home Telehealth (HT) infrastructure for patients with uncontrolled type 2 diabetes despite receiving routine care.
The method used for the study comprised of Advanced Comprehensive Diabetes Care (ACDC) which was a 6-month telehealth intervention for patients with poor glycemic control (varied by site, typically ≥8.5%), and was established as effective in a prior randomized trial.
ACDC comprised of telemonitoring, self-management support, and clinician-guided medication management, and was delivered by clinical HT nurses using standard HT equipment. From 2017-2019, the team implemented and evaluated ACDC at 5 rural VHA sites with guidance from the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework.
During this time period, 30-100% of eligible patients at each site were enrolled in ACDC, summing up to a total of 125 patients. The results depicted that across sites, mean HbA1c improved from 9.25% at baseline to 7.89% at 6 months, a benefit that persisted at 12 and 18 months. Implementation at each site was acceptable, with an average of 8-10 of 12 scheduled ACDC summed up to its completion. Quantifiably, ACDC enhanced patient engagement and awareness of glycemic control, while moderately increasing workload for providers.
Conclusion: When strategically designed to use existing infrastructure, intensive telehealth interventions are conducive to implementation and produce sustained improvements in glycemic control among rural patients.
Acute Declines in EGFR during Treatment with Canagliflozin and Its Implications for Clinical Practice: Insights from CREDENCE
Insights from Credence were shared by Megumi Oshima at the 80th Scientific Sessions of ADA , A Virtual Experience on 12th June 2020. Canagliflozin (CANA) not only does slow the progression of Chronic Kidney Disease (CKD) but also induces a reversible acute decline in estimated glomerular filtration rate (eGFR), which is believed to be a hemodynamic effect.
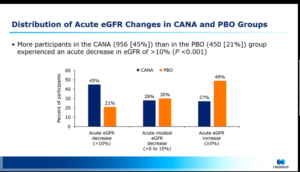
The post-hoc study of the CREDENCE trial comprised of 4289 patients with type 2 diabetes and CKD who had eGFR measured at both baseline and 3rd week. Categorically, patients were divided in 3 groups as per percentage decline in eGFR at week 3: >10%, ≤10% to >0%, and ≤0%. Logistic regression model was used to evaluate baseline characteristics associated with acute eGFR declines >10%. Linear mixed effects models and Cox regression were used to study the long-term eGFR decline and safety outcomes in each eGFR decline category.
The results for the study demonstrated that more participants in the CANA vs Placebo (PBO) group had an acute eGFR decline >10%. It was also observed that >30% decline occurred infrequently with CANA and 39 with PBO. Only in the CANA group, older age and history of heart failure were associated with a higher and lower likelihood of an acute eGFR decline >10%. Following the initial eGFR change, long-term eGFR trajectories as well as overall safety profiles were similar across eGFR decline categories Consistent results were observed when other decline thresholds (>20%) were used and in subgroup analysis by baseline eGFR (30-<45, 45-<60, and 60-<90 ml/min/1.73 m2).
Conclusion: Although acute eGFR declines >10% occurred in nearly half of all patients following initiation of CANA, the benefit of CANA compared with placebo was observed regardless of the acute eGFR decline. The study also demonstrated similar safety profiles.
How Early after Treatment Initiation Are the CV Benefits of Empagliflozin Apparent? A Post Hoc Analysis of EMPA-REG OUTCOME
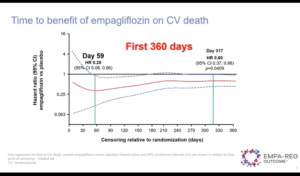
The EMPA-REG OUTCOME trial was presented at 80th Scientific Sessions of ADA by Subodh Verma and colleagues. The trials was conducted in in patients with type 2 diabetes (T2D) with established atherosclerotic cardiovascular disease (ASCVD). In the trial, empagliflozin reduced the risk of hospitalization for HF (HHF) by 35%, CV death/HHF by 34% and CV death by 38%with an early separation of the cumulative incidence curves.
The study aimed to explore the time point after randomization the benefits became apparent. In the study, 7020 patients were treated with empagliflozin 10, 25 mg or placebo. The authors expressed time trajectories for the effect of pooled empagliflozin doses vs. placebo on HHF, CV death/HHF and CV death based on Hazard Ratios (HRs) (95% CI), and calculated the HR on the day the effect reached significance using Cox proportional hazards models. The reduction in risk with empagliflozin vs. placebo reached significance at day 17 for HHF and day 27 for CV death/HH, p=0.0445], and sustained significant throughout follow-up (Figure)
Conclusion: The benefit of empagliflozin in reducing the risk of HHF, CV death/HHF and CV death emerged within weeks after treatment initiation in EMPA-REG OUTCOME. The earliest effect appeared to be on HHF.
Empagliflozin Delays Need for Insulin Initiation in Patients with Type 2 Diabetes and Cardiovascular Disease: Findings from EMPA-REG OUTCOME
Muthiah Vaduganathan and Team presented findings for EMPA-REG OUTCOME at the 80th Scientific Sessions of ADA. Since Insulin in T2D is linked with hypoglycemia & weight gain it requires training and can be expensive which makes it less patient compliant. As per EMPA=REG Outcome, 7020 patients were treated with empagliflozin (EMPA) 10, 25 mg, or placebo (PBO) with medican followup being 3.1 yr. Post 12 weeks, changes in background glucose-lowering therapy were permitted. The team assessed, treatment effects of pooled EMPA vs. PBO on time to new initiation of insulin among insulin-naïve patients and time to total daily insulin dose increase by >20% among insulin-treated patients. In 3633 (52%) insulin-naïve patients, EMPA reduced need for insulin use vs. PBO by 54%.In 3387 (48%) patients using insulin at baseline, EMPA reduced need for a >20% increase in insulin dose by 57%.
Conclusion: In T2D patients and CVD, EMPA markedly and durably delays the need for insulin initiation, more so in those recently diagnosed, and reduces need for large dose increases in those already using insulin.

Risk of Cardiovascular Outcomes in Diabetes Patients following the Addition of SGLT2 Inhibitors vs. Sulfonylureas to Baseline GLP-1RA Therapy
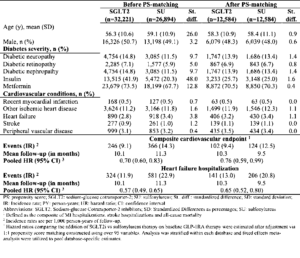
According to a presentation by Chintan Dave at the 80th Scientific session of ADA adding SGLT2i to GLP-IRA therapy confers greater CV benefit compared to adding sulfonylureas. The author conducted the study wherein, patients adding either SGLT2i or sulfonylureas to baseline GLP-1RA were identified within 3 U.S. datasets (2013-2018) and were 1:1 propensity score matched (PSM) adjusting for >95 baseline covariates. Primary outcomes of the study were: 1) a composite CV endpoint (comprised of myocardial infarction, stroke, or all-cause mortality) and 2) heart failure hospitalization. The author estimated pooled hazard ratios & 95% confidence intervals. Among 12,584 1:1 PSM pairs, there were 102 composite CV events among SGLT2i initiators compared to 124 events among sulfonylurea initiators, with an adjusted HR of 0.76 over a mean 10-month follow-up. For the outcome of heart failure hospitalization, there were 141 (IR = 13.0) v 206 (IR = 20.8) events, with an adjusted HR of 0.65.
Conclusion: The study concluded that adding SGLT2i to GLP-IRA therapy confers greater CV benefit compared to adding sulfonylureas, with a magnitude of risk reduction comparable to the benefit seen in CV outcome trials of SGLT2i vs. usual care in patients with minimal background GLP-IRA use.
Comparative Effectiveness of SGLT2 Inhibitors vs. GLP-1 Agonists
NSIYA POONAWALLA, et al. presented about Comparative Effectiveness of SGLT2 Inhibitors GLP-1 over Agonists at the 80th Scientific session of ADA. The author compared sodium-glucose co-transporter-2 (SGLT2) inhibitor and glucagon-like peptide-1 (GLP-1) agonist therapeutic drug classes on cardiovascular (CV) outcomes, treatment persistence/discontinuation, healthcare utilization and costs. new users of SGLT2 inhibitors or GLP-1 agonists from January 2015 to June 2017 were identified using medical and pharmacy claims data from the Humana Research Database.
Patients eligible for the study were 19-89 years, enrolled in a Medicare Advantage Prescription Drug or fully-insured commercial health plan, with at least 12 months of continuous enrollment pre- and post-index date. Baseline characteristics were balanced using propensity score matching (1:1). Cox proportional hazards models were used to compare CV outcomes and treatment discontinuation. Healthcare resource utilization and costs were compared using Wilcoxon signed rank test.
A total of 5,507 patients were included in each treatment group. Patients indexed to use GLP-1 agonists were more likely to discontinue treatment (hazard ratio 1.15, 95% confidence interval 1.10-1.21) and more likely to encounter an inpatient hospitalization (14.4% vs. 11.9%, P<0.001) or emergency department visit (27.4% vs. 23.5%, P <0.001) compared to patients indexed on SGLT2 inhibitors. The risk of primary composite CV outcome (myocardial infarction or stroke or mortality) and secondary CV outcome (heart failure or death) was similar across cohorts. The study results depicted average per-person per-month total cost, medical and pharmacy costs were higher for the GLP-1 agonist cohort compared to the SGLT2 inhibitor cohort.
Conclusion: Although differences in extra-glycemic CV benefits were not established, these findings may prove be useful to providers and payers, as this real-world data showed SGLT2 inhibitors were associated with longer treatment persistence and reduced healthcare utilization and costs relative to GLP-1 agonists.
Antihypertensive Effect of Azilsartan in Newly Diagnosed Stage 2 Diabetic Hypertensives
HARIBALLAV MAHAPATRA et.al. presented a study at the the 80th Scientific session of ADA. The study aimed to study for Blood pressure (BP) lowering effect of Azilsartan (AZST) monotherapy in newly diagnosed stage 2 hypertensives with a history of T2DM. The authors also analysed its effects on blood glucose and renal indices.
The participants for the study were T2DM Subjects who had attended a specialized diabetes clinic and were diagnosed with HTN for the first time. Mean of 3 BP readings was considered for analysis after due consent. Stage 2 hypertensive patients were administered AZST 40mg once daily. A repeat BP enrollment was conducted post 3 months. Subjects underwent serum creatinine, Na+, K+, fasting (FPG) and postprandrial (PPG) plasma glucose measurements at baseline and after 3moths’ of enrolment. The comparative parameters were BP lowering efficacy and changes in plasma glucose and renal parameters at baseline and after 3months of AZST therapy.
The inclusion criteria met by 474 subjects while Mean age and duration of diabetes were 53.3 years and 7.03 years respectively. Mean SBP dropped from 152.7 mm Hg to 136.4 mm Hg with AZST therapy and the post treatment DBP declined to 79.6 mm Hg from its baseline value of 90.5. The mean baseline FPG and PPG were 158.8 mg/dL and 237.3 mg/dL in order. There was a non-significant decline in plasma glucose, but no changes were seen in eGFR or electrolytes. No adverse event was reported.
Conclusion: Azilsartan monotherapy was found to be safe and effective in managing newly diagnosed stage 2 hypertension in type 2 diabetes without hampering renal function.
Relationship between SGLT2 Inhibitor, ACEI/ARB, and Pioglitazone to Renal Function in Patients with Type 2 Diabetes Mellitus
TAO-CHUN LEE presented relationship between various classes of antidiabetics at the 80th scientific session of ADA. The study aimed to discuss the interplay between SGLT2i, ACEI/ARB and pioglitazone to changes of renal function in patients with type 2 diabetes (T2DM). Patients with T2DM who initialized SGLT2i over six months and had at least two serum creatinine examinations were included in the study. The author categorized the participants into three groups according to their ACEI/ARB and pioglitazone status. GEE was used to analyze significant factors for subsequent EPI changes.
A total of 253 patients with T2DM were included in the study whose SGLT2i treatment duration was 23.7±9.6 months and serum creatinine was followed 4.1± 1.6 times during the study period. After SGLT2i initiation, A1C reduced 0.5%, body weight decreased 2.2 kg, systolic blood pressure dropped 6 mmHg and LDL-C lowered 7 mg/dl; however, EPI showed a J-curve improvement as treatment duration increased.
To disclose the interaction between ACEI/ARB and pioglitazone in the SGLT2i users, the authors categorized the participants into three groups, and the number of patients in the ACEI/ARB(-)/pioglitazone(-), ACEI/ARB(+)/or pioglitazone(+) and ACEI/ARB(+)/pioglitazone(+) group was 83, 133 and 37, respectively. In the consideration of the effect of age, gender, body weight, A1C, albuminuria status, treatment duration, systolic and diastolic blood pressure and LDL-C, the author found SGLT2i users who simultaneously were under ACEI/ARB and pioglitazone therapy had a significant renal function deterioration by 5.77 mL/min/1.73m2 (P=0.011) as compared with other two group.
Conclusion: The study suggested that the encouraging renal effect brought by SGLT2i may be lessened in patients with T2DM who simultaneously under ACEI/ARB and pioglitazone treatment.
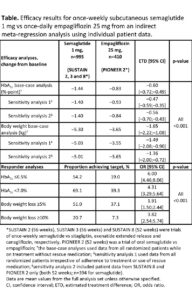
Once-Weekly Semaglutide 1mg vs. Empagliflozin 25mg as Add-On to Metformin Monotherapy in Patients with Type 2 Diabetes: A Meta-regression Analysis of Individual Patient Data
ILDIKO LINGVAY, et.al. presented a study on the molecule Semaglutide at the 80th scientific session of ADA. As there are no published head-to-head trials of once-weekly semaglutide 1 mg vs. once-daily empagliflozin 25 mg in type 2 diabetes (T2D) the authors indirectly compared the pooled semaglutide arms in SUSTAIN 2, 3 and 8 with the empagliflozin arm in PIONEER 2 using individual patient data, for those on metformin monotherapy. Although all trials had similar inclusion criteria and duration, meta-regression analyses adjusting for effect modifiers and prognostic factors further controlled for differences in design and population. Mean baseline characteristics of age, diabetes duration, BMI, HbA1c were similar with semaglutide and empagliflozin. Semaglutide reduced mean HbA1c by -1.4 vs. -0.8%-point with empagliflozin , and body weight by -5.3 vs. -3.7 kg with empagliflozin. A significantly greater proportion of patients on semaglutide vs. empagliflozin also achieved HbA1c targets and clinically relevant weight-loss targets (Table).
Conclusion: In this indirect comparison by the author, pooled semaglutide 1 mg provided significantly greater HbA1c reduction and body weight loss compared with empagliflozin 25 mg in patients with T2D on metformin monotherapy.
Remogliflozin in Indian T2DM Patients: A Real-World Study
A real world study on Remogliflozin was presented by SUPRATIK BHATTACHARYA et.al at the 80th scientific session of ADA. Since Remogliflozin is new SGLT2i approved in India it is provident to evaluate its effectiveness and tolerability in real world setting. In the observational study, retrospective data from EMR of tertiary care hospital in Kolkata, India was collected. Inclusion criteria for patients was the ones who were uncontrolled (HbA1c>7.5) on dual therapy (Metformin + DPP-4 inhibitors/Sulphonylureas) and subsequently treated with Remogliflozin for period of at least 6 months.
Exclusion criteria was patients with active UTI at baseline, receiving injectable antidiabetic drugs or recorded eGFR <45 mL/min.The effectiveness was assessed in terms of change in HbA1c, FBS, PPBS, body weight after 3 and 6 months of treatment. Safety parameters were assessed by adverse events recorded in medical records in terms of abnormal symptoms, signs or laboratory reported values.
The results of the study were obtained by medical records of 120 adult patients with T2DM. At end of 6 months of add on Remogliflozin treatment , the mean change from baseline in HbA1c, FBS, PPBS, Body weight and BMI was -1.3±0.4%, -17.2±9.2 mg/dL, -56.4±18.4 mg/dL, -4.2±1.8 kg, -1.8±0.6 kg/m2.SUs patients had more reduction in body weight in comparison to DPP-4 Inhibitors. (-5.2 vs. -2.9kg). At end of 6 months, HbA1c reduced <8% in all patients while 66% achieved target HbA1c of 7%. No events of hypoglycaemia or electrolyte disturbances were reported. Combined incidence of UTI and GTI was 5.8% which were mild. None of the patients on Remogliflozin were discontinued for adverse events.
Conclusion: Addition of Remogliflozin to existing OHA regimen is observed to be an effective, safe and tolerable option in management of Indian patients with T2DM.

