
A retrospective cohort study published in Cureus on January 31, 2026, assessed the effectiveness of a specialized vascular risk clinic in Portugal in controlling major modifiable cardiovascular (CV) risk factors. Conducted between January 2022 and April 2023 (16 months), the analysis included 229 consecutive patients referred to the clinic, predominantly those with established atherosclerotic CV disease (ASCVD), diabetes, or high/very high CV risk per ESC/EAS guidelines. The multidisciplinary approach involved cardiologists, endocrinologists, nutritionists, and nurses, emphasizing intensive lifestyle counseling (diet, exercise, smoking cessation) alongside evidence-based pharmacotherapy titration (statins, antihypertensives, antiplatelet agents, GLP-1 agonists/SGLT2 inhibitors where indicated).
Gestational diabetes mellitus (GDM) is a common pregnancy complication associated with adverse maternal and fetal outcomes, including macrosomia, preterm birth, preeclampsia, and increased future diabetes risk. Myo-inositol, an insulin-sensitizing nutrient, has shown promise in prior studies for reducing GDM incidence in high-risk groups (e.g., those with family history of type 2 diabetes, obesity, or PCOS). This pilot trial, titled “Myo-Inositol for the Prevention of Gestational Diabetes Mellitus (MiGDM),” was designed as a randomized, double-blind, placebo-controlled study to preliminarily evaluate myo-inositol supplementation’s effects on GDM prevention and broader fetal/maternal outcomes.
Acute ischemic stroke (AIS) remains a leading cause of disability and death worldwide. The 2026 Guideline for the Early Management of Patients With Acute Ischemic Stroke, from the American Heart Association/American Stroke Association (AHA/ASA), replaces the 2018 guideline and 2019 focused update. It incorporates evidence from randomized controlled trials, meta-analyses, and observational studies published through early 2025, addressing prehospital systems, emergency evaluation, reperfusion therapies, supportive care, in-hospital complications, and early secondary prevention for adults, with new guidance extending to select pediatric cases.
On January 23, 2026, OKYO Pharma Limited (Nasdaq: OKYO), a clinical-stage biopharmaceutical company focused on therapies for neuropathic corneal pain (NCP) and inflammatory eye diseases, announced that the U.S. Food and Drug Administration (FDA) has authorized a single-patient expanded access Investigational New Drug (IND 176297) application—commonly known as compassionate use—for urcosimod (0.05%). The IND was submitted by Pedram Hamrah, MD, at the University of South Florida, to treat a patient suffering from severe NCP who has exhausted available therapeutic options, with no FDA-approved treatments currently existing for this condition.
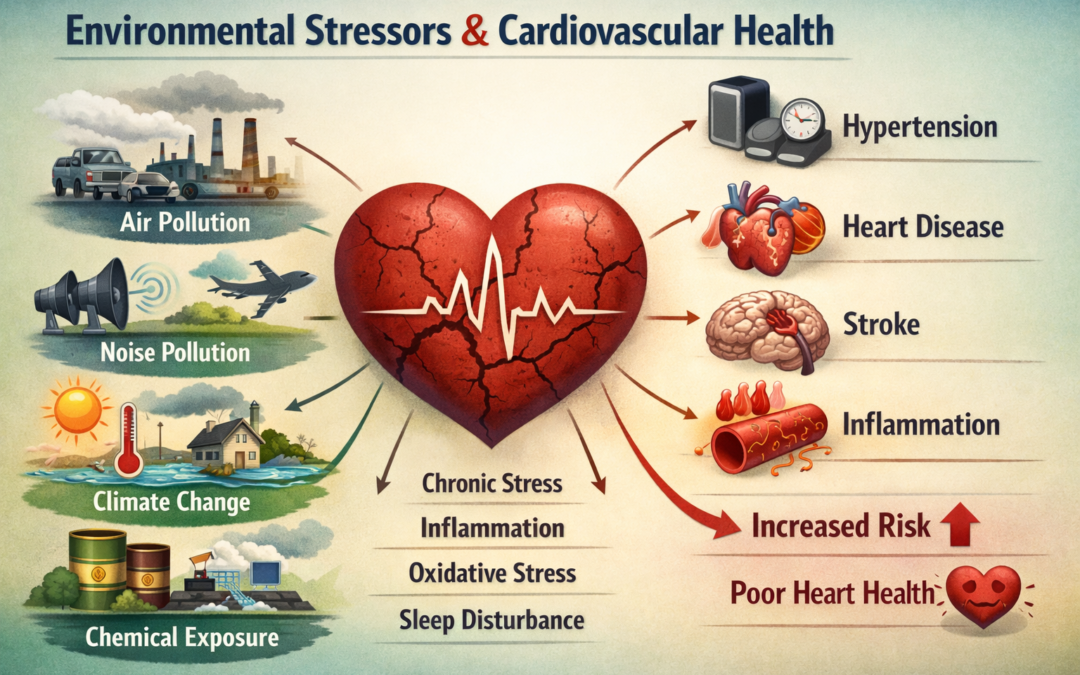
Non-communicable diseases (NCDs) account for 70% of global mortality, claiming over 38 million lives each year, with cardiovascular disease (CVD) as the leading contributor. While conventional risk factors like smoking, hypertension, and poor diet remain critical, emerging evidence highlights the escalating role of ubiquitous environmental risk factors (ERFs) in driving the rise of NCDs, particularly CVD. These interconnected anthropogenic exposures—air pollution, noise and light pollution, chemical and plastic contamination, water and soil pollution, and climate-related hazards—exert cumulative and compounding effects on cardiovascular health. They operate through shared pathophysiological mechanisms, including oxidative stress, systemic inflammation, autonomic nervous system imbalance, and endothelial dysfunction, amplifying overall risk beyond traditional factors.






