
On September 25, 2025, the U.S. Food and Drug Administration (FDA) announced the approval of leucovorin calcium tablets for the treatment of cerebral folate deficiency (CFD), a neurological disorder characterized by impaired folate transport into the brain, resulting in developmental delays, autistic features (e.g., social communication challenges, sensory processing issues, repetitive behaviors), seizures, and motor coordination difficulties.

On September 22, 2025, the U.S. Food and Drug Administration (FDA) announced it is initiating a label change for acetaminophen, commonly known as Tylenol, to reflect evidence suggesting a potential association between its use during pregnancy and increased risks of autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) in offspring.
On September 19, 2025, the U.S. Food and Drug Administration (FDA) approved Merck’s KEYTRUDA QLEX™ (pembrolizumab and berahyaluronidase alfa-pmph) injection for subcutaneous use in adults across 38 solid tumor indications previously approved for KEYTRUDA® (pembrolizumab), marking it as the first subcutaneously administered immune checkpoint inhibitor.
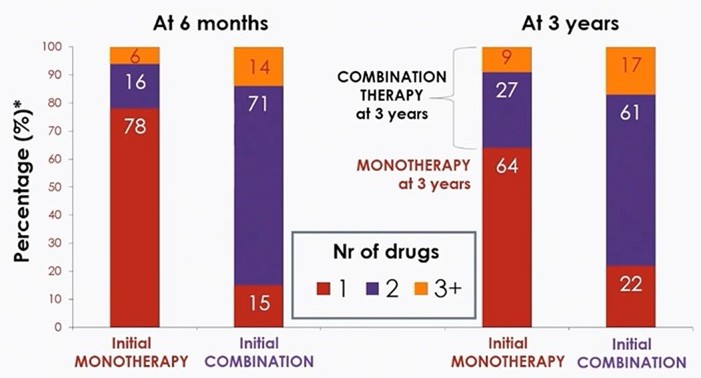
Hypertension remains a leading cause of cardiovascular morbidity and mortality worldwide, with suboptimal control and poor medication adherence posing significant challenges, particularly for cardiometabolic patients in 2025. Single-pill combinations (SPCs), which integrate multiple antihypertensive drugs into a single dose, have emerged as a transformative approach to address these issues. By simplifying treatment regimens, SPCs significantly improve patient adherence compared to multi-pill regimens, which often lead to missed doses and therapeutic failure.
Heart failure with preserved ejection fraction (HFpEF) represents a significant clinical challenge due to limited effective pharmacological treatments. Sodium-glucose co-transporter 2 (SGLT2) inhibitors, such as empagliflozin and dapagliflozin, have emerged as a novel therapeutic approach. This systematic review synthesizes evidence from 10 randomized controlled trials (RCTs) involving diverse HFpEF patient populations, including those with comorbidities like diabetes and chronic obstructive pulmonary disease.






